Mind Blowing: Biological Robots Repair Neurons When Free
Scientists find that, when left to their own devices, our lung airway cells form motile clusters that promote the repair of damaged neuronal tissue.
Highlights:
- When lung cells are grown in the absence of a prison-like matrix, they form fully-motile spherical biological robots called anthrobots.
- When placed into areas of damaged neuronal tissue, the anthrobots promote the growth of new neuronal tissue, suggesting their reparative properties.
As published in Advanced Sciences, researchers from Tufts University unveil their discovery of anthrobots — biological robots derived from human cells. They showed that anthrobots composed of lung cells are fully motile and able to promote the growth of neuronal tissue.
Breaking The Mold
Never did anyone imagine stationary cells could serve beneficial purposes beyond their normal function. Lining the airways of our lungs are stationary cells called epithelial cells that help neutralize harmful substances from inhaled air. Providing a protective barrier was once thought to be the only function of these lung airway epithelial cells. However, Tufts University researchers have broken the mold of this tradition.
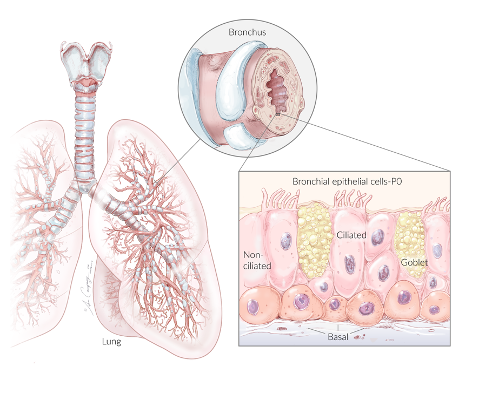
Scientists have previously reconstructed lung airway lining in a laboratory dish using a gel-like matrix that holds the cells in place like a mold. In contrast, Tufts researchers grew lung airway cells in the absence of such a mold-like matrix. Instead, they used a water-based medium that allowed the cells to move freely. This resulted in fully-motile spherical anthrobots, propelled by outwardly projecting cilia.
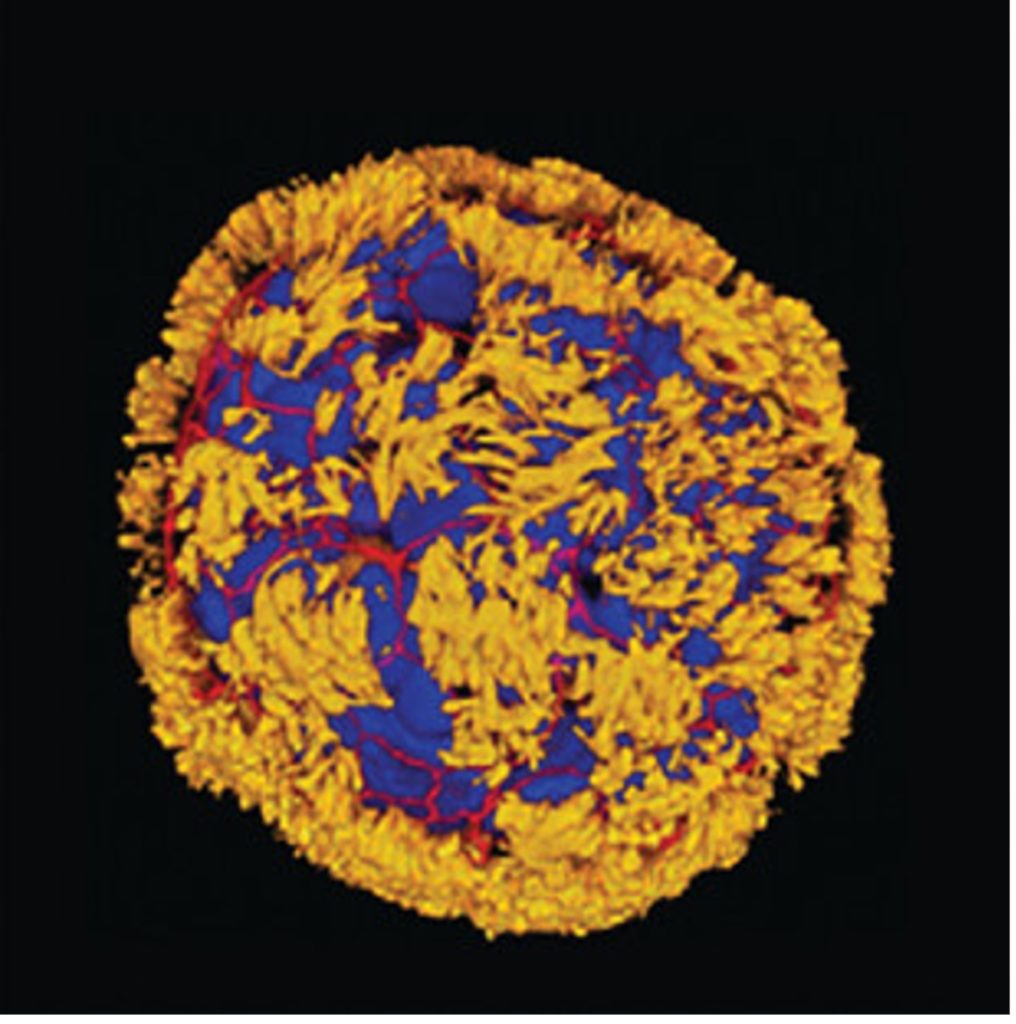
Bridging the Gap
The Tufts researchers next determined if the anthrobots could manipulate other tissues. To do so, they first grew a thin layer of neuronal tissue in a dish. They then scratched the tissue with a plastic tip to cause damage. Microscopically, the scratch looks like a giant gap that separates the neuronal tissue. The researcher subsequently placed multiple anthrobots across this gap. As a result, new neuronal tissue grew where the antrobots had been placed, suggesting they had begun repairing the tissue.

Biological Robots, Bioelectricity, and Regenerative Medicine
The Tufts University researchers, led by Dr. Micheal Levin, have made multiple mind-blowing discoveries. This includes using drugs to regenerate the legs of adult frogs, which are animals that normally cannot regenerate limbs. The team accomplished this by finding how cells use electricity to form new structures. Remarkably, it seems that manipulating our cells’ electrical properties could lead to the regeneration of organs and tissues.
This technology, along with the tissue-repairing biological robots has far-reaching implications for regenerative medicine — replacing damaged tissues to restore normal function. Furthermore, considering that aging is essentially the result of degenerating organs and tissues, this potentially groundbreaking regenerative technology could be used to combat aging.

