Aging Might Be Reversible — Fixing the Miscommunications in Cells with NAD+
Miscommunication between mitochondria and the nucleus accelerates aging, but NAD+ supplementation helps restore the conversation and reverses signs of aging.
Our cells are like intricate machinery that rely on different components’ cooperation and communication, especially between the central command center and the powerhouse — the nucleus and mitochondria. Scientists at Harvard University discovered that the communication breakdown between the two organelles in mice leads to accelerated aging. On the bright side, it might be reversible through a natural compound produced by the body — nicotinamide adenine dinucleotide (NAD+).
“The aging process we discovered is like a married couple — when they are young, they communicate well, but over time, living in close quarters for many years, communication breaks down,” said senior author of the study, David Sinclair of Harvard Medical School in a statement. “And just like with a couple, restoring communication solved the problem.”
Mitochondria are tiny structures in complex cells that produce adenosine triphosphate (ATP), the biological fuel for metabolism. About 2 billion years ago, mitochondria’s bacterial predecessors integrated itself into host cells and specialized in energy production. Today, mitochondria still carry a small amount of their own DNA and play a crucial role in facilitating cell metabolism. Scientists have recognized mitochondria dysfunction as one of the hallmarks of aging, which leads to age-related conditions such as cancer, diabetes and Alzheimer’s disease.
NAD+ Supplementation Restores Mitochondrial Function
Researchers have known that aging is associated with a progressive decline in mitochondrial function. However, to the team’s surprise, while the level of mitochondrial proteins from the nucleus remained normal in older mice, those encoded by the mitochondrial DNA were significantly reduced.
Further investigation showed that one of the root causes might be the declining levels in NAD+ molecules. The essential molecule acts as a shuttle bus that carries information between the nucleus and mitochondria, coordinating the communication. But as we age, the levels of NAD+ decline for reasons that scientists still don’t understand.
What Sinclair’s team does know is that NAD+ is crucial for supporting the function of SIRT1, a protein that safeguards the communications between the nucleus and mitochondrial genome from an interfering molecule — HIF-1. Without the NAD+-dependent SIRT1 to keep an eye on HIF-1, it roams free, disrupting the conversation. The researchers found that this disruption decreases energy production in cells and accelerates the process of aging. Not only is this the first time nuclear-mitochondrial communication during aging has been described, but the study also showed that the phenomenon is “reversible.”
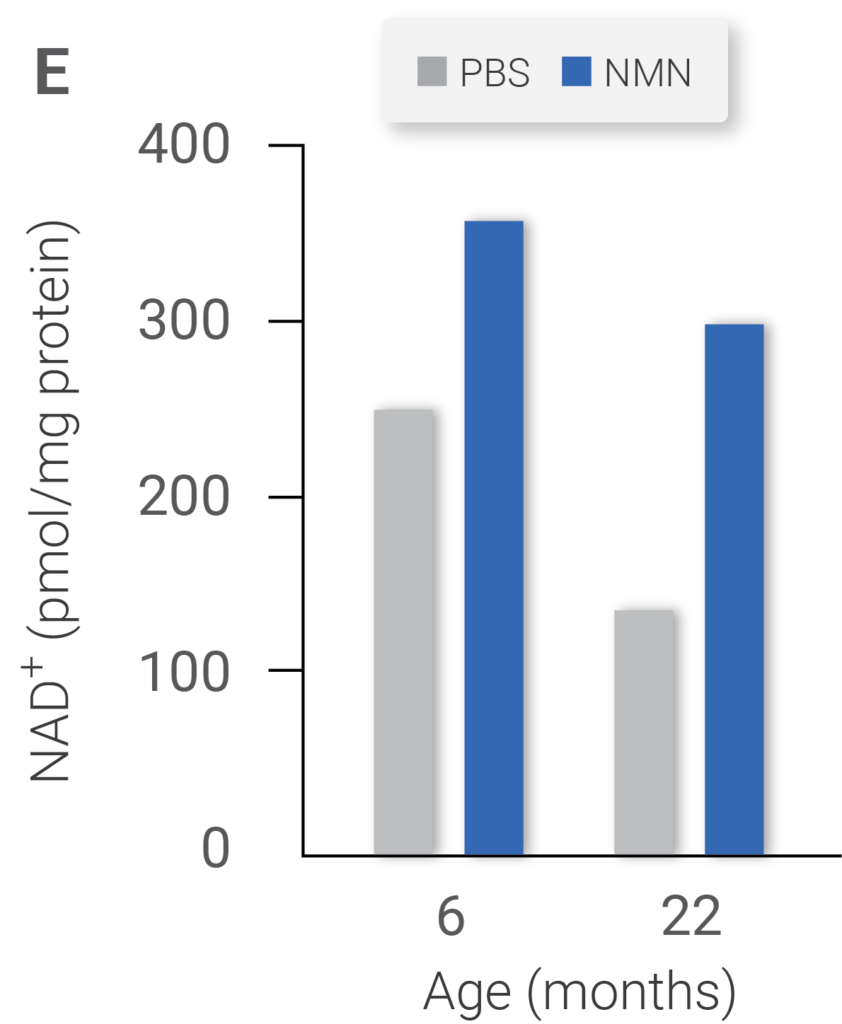
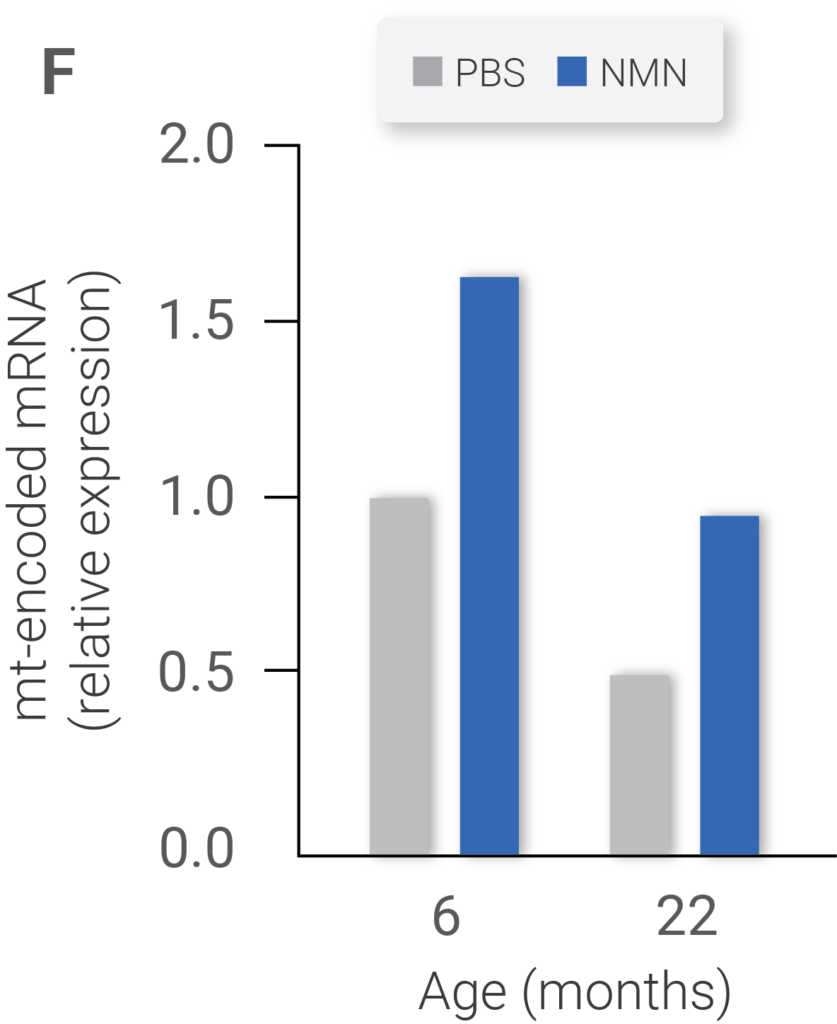
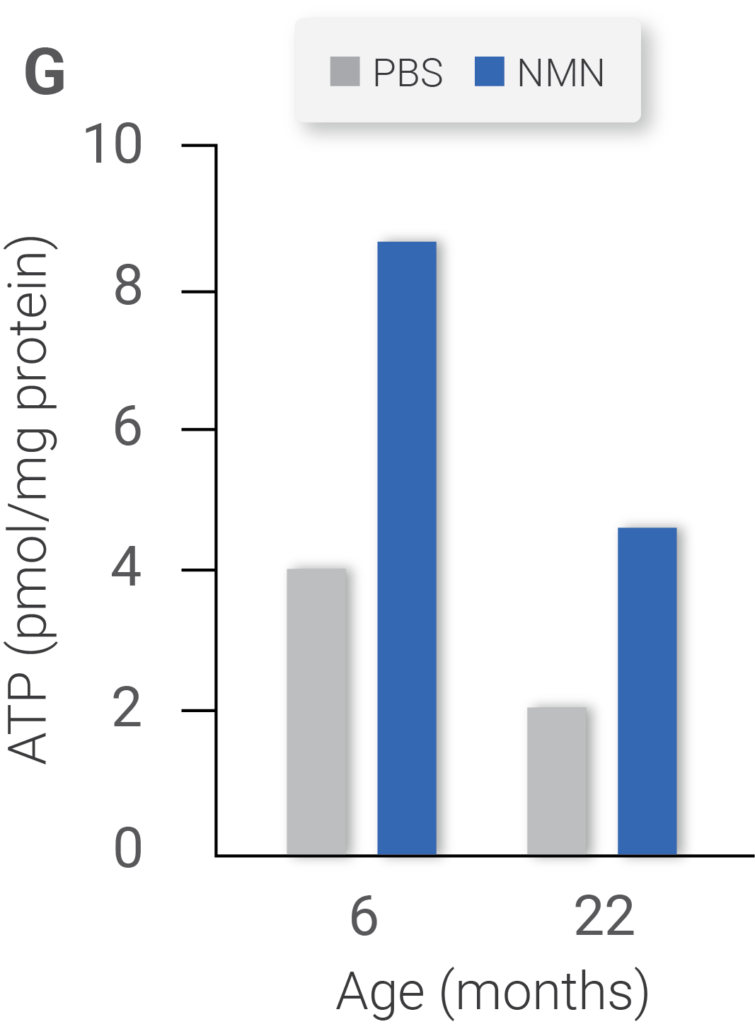
NAD+ levels (Figure E) in 6- and 22-month-old mice treated with vehicle (PBS) or NMN. Expression of mitochondrially encoded genes of the same cohort (Figure F). ATP content of same cohorts (Figure G) (Gomes et al., 2013)
They found that by injecting NAD+ supplement into 2-year-old mice, they could repair the cross-genome communication network and restore mitochondrial function. One week of treatment was sufficient to bring biochemical markers of muscle health to levels similar to 6-month-old mice. It would be like converting a 60-year-old human to a 20-year-old in specific areas.
Injecting nicotinamide mononucleotide (NMN), an NAD+ booster, increased the NAD+ content in both young and old mice. In aged mice that received treatment, the level of NAD+ about doubled those untreated. The researchers also observed animals treated with NMN to have higher ATP levels, indicating an increase in mitochondria activity, in which the cells produce more energy.
“There’s clearly much more work to be done here, but if these results stand, then many aspects of aging may be reversible if caught early,” said Sinclair. The team suggested that developing small compounds that prevent HIF-1’s meddling in cellular communication or increasing NAD+ levels may be an effective therapy for treating aging in the future.

