Apigenin Ameliorates Kidney Injury in Diabetic Rats
Blocking enzymes that deplete NAD+ may treat human metabolic diseases like diabetic kidney disease
Highlights
- CD38 levels are increased in the damaged kidneys of diabetic rats
- Apigenin, an inhibitor of CD38, has antioxidant properties that mitigate diabetes-induced kidney injury
Diabetic kidney disease is a serious diabetes-related complication and the leading cause of end-stage kidney disease. Since this kidney damage, often found in type 2 diabetes patients, is induced by multiple metabolic risk factors, including hyperglycemia, hypertension, dyslipidemia, and obesity, the treatment approach is usually to manage all of these metabolic risk factors. But even when patients try to manage these multiple factors, the therapy is often insufficient to suppress the progression of diabetic kidney disease, and there is still a residual risk of progression to end-stage kidney disease.
New research published in Aging by Ogura and colleagues from Kanazawa Medical University in Japan show that a plant-based compound significantly reduced kidney injuries, including kidney cell damage and pro-inflammatory gene activity in diabetic rats. This compound called apigenin is present in vegetables, fruits, herbs, and plant-based beverages and works by inhibiting the enzyme CD38, which is a cell-depleting consumer of the vital molecule NAD+. Here, apigenin showed antioxidative properties in the kidneys of diabetic rats, leading to the amelioration of diabetes-induced kidney injury.
“We believe that these findings may lead to a novel strategy for the treatment of diseases characterized by an imbalance in NAD+ metabolism, including diabetic kidney disease,” proposed the authors.
The link between NAD+ levels and diabetic kidney disease
A large number of mitochondria, the power-generating structures of cells, reside in kidney cells to meet the high energy demand necessary for the reabsorption of nutrients. These mitochondria are a major source of cell damaging molecules called reactive oxygen species (ROS) in the kidney, which show exacerbated levels in diabetes. So, protecting kidney cells against mitochondrial oxidative stress in diabetic kidneys might serve as a therapeutic strategy to preserve the renal function.
Mitochondrial oxidative stress occurs due to the imbalance between ROS production and anti-oxidative capacity. Mitochondrial oxidative stress is induced by decreased activity of antioxidant enzymatic activities, which is linked to increased gene activity of the NAD+ degrading enzyme CD38 in the kidney and decreased NAD+ levels. Previous reports have shown that mice lacking CD38 have higher NAD+ levels and are protected against high fat diet-induced obesity and metabolic syndrome.
Activity of CD38 increases during aging, and this is associated with age-related decline in NAD+, reduction in activity of an enzyme called Sirt3, and mitochondrial dysfunction in liver, adipose tissues, and skeletal muscles. But whether increased CD38 activity is involved in the pathogenesis of diabetic kidney disease caused by mitochondrial oxidative stress has been unclear.
CD38 inhibition by apigenin mitigates kidney damage in diabetic rats
To evaluate the role of CD38 in diabetic kidney disease, Ogura and colleagues treated lean and diabetic fatty rats with the CD38 inhibitor apigenin (20 mg/kg) or control saline solution orally. Apigenin is present in vegetables (parsley, celery, and onions), fruits (oranges), herbs (chamomile, thyme, oregano, and basil), and plant-based beverages (tea, beer, and wine). A previous study has shown that apigenin inhibits CD38, thus increasing NAD+ levels, and improving glucose and lipid homeostasis in obese mice. However, there have been few reports evaluating the effect of apigenin on diabetic kidney disease.
In this study, the kidneys of the diabetic fatty rats treated with saline showed major increases in the gene and protein levels of CD38 in the kidneys, but administration of apigenin significantly reduced these CD38 levels. Along these lines, an indicator of available NAD+ levels known as the NAD+/NADH ratio was significantly decreased in the diabetic fatty rats treated with saline compared to lean rats treated with saline. But apigenin increased the NAD+/NADH ratio in diabetic fatty rats.
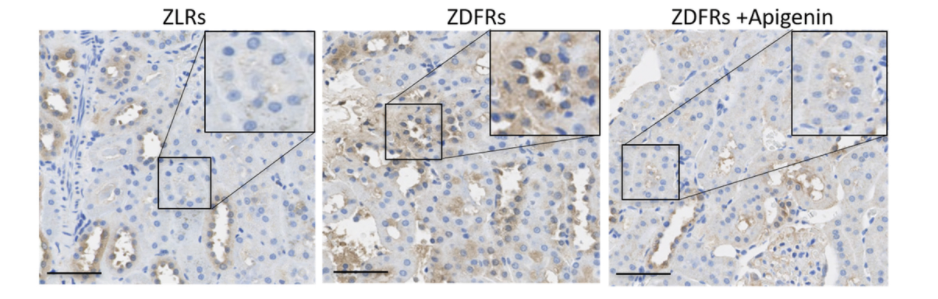
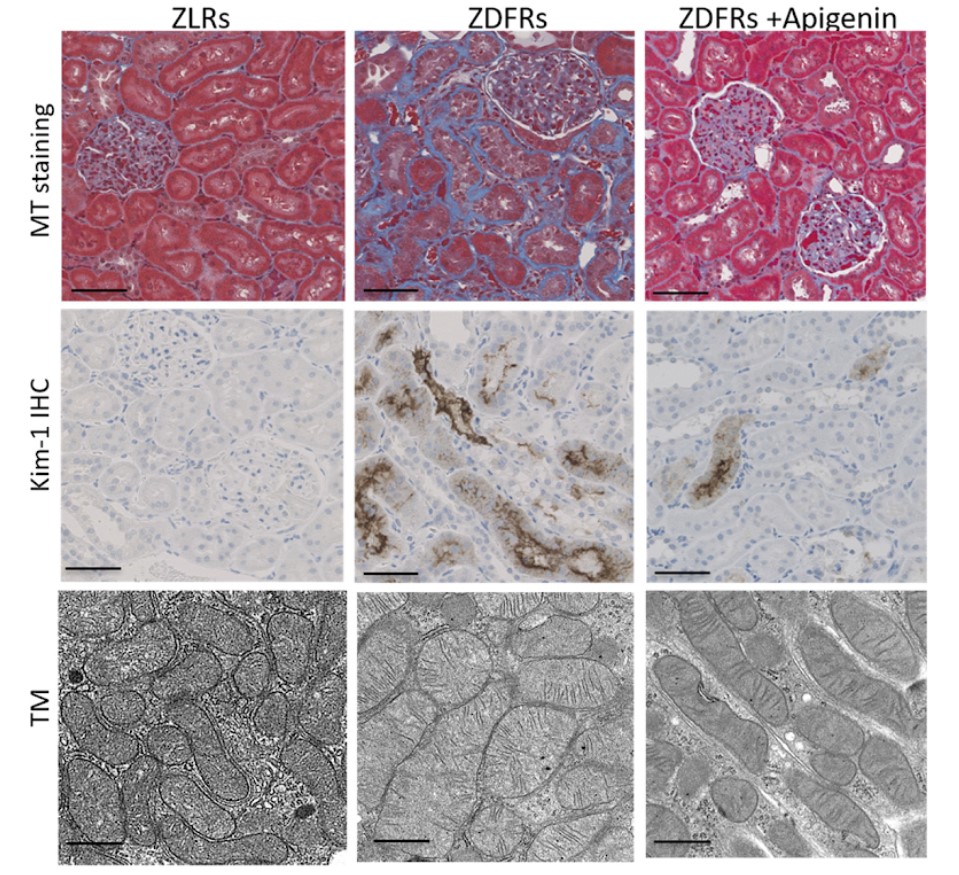
Although there was no significant change in whole body and kidney weight among the four groups of rats, the diabetic fatty rat group treated with saline exhibited fibrosis of the kidney and an altered mitochondrial morphology, such as mitochondrial swelling, in the proximal tubular cells. These obese rats also had an increased gene activity in the outer portion of the kidney called the renal cortex of collagen III and anti-kidney injury molecule-1 (Kim-1), and of the pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). Importantly, administration of apigenin ameliorated these alterations.
Apigenin rebalances NAD+/NADH levels to rescue antioxidant enzyme activity
The enzymes superoxide dismutase 2 (SOD2) and isocitrate dehydrogenase 2 (IDH2) manage oxidative stress, which is critical to the health of all of our cells, and the kidney is no exception. When the activity of these antioxidant enzymes decreases, there is an increase in oxidative stress. The function of these antioxidant enzymes is thought to be linked to the NAD+/NADH ratio and activity of another enzyme dependent on NAD+ called Sirt3 — when NAD+ isn’t available, SIRT3 can’t function. SIRT3 is thought to activate both SOD2 and IDH2
Ogura and colleagues found that the levels of inactive IDH2 and SOD2 were significantly increased in the diabetic fatty rats treated with saline compared to lean rats treated with saline. The researchers say that this indicates reduced Sirt3 activity in diabetic rats. However, apigenin promoted the activation of IDH2 and SOD2, indicating that apigenin increases the activity of SIRT3 that is dependent on NAD+ in the kidneys of diabetic rats.
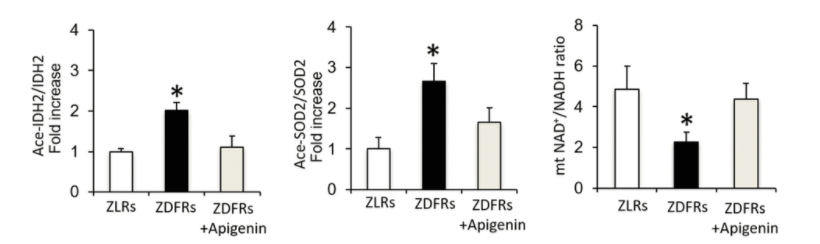
“Here, we show for the first time that CD38 plays a crucial role in mitochondrial oxidative stress by reducing the NAD+/NADH ratio and Sirt3 activity in the kidneys of type 2 diabetic rats,” concluded the authors. Apigenin ameliorates the diabetes-induced renal tubular injury by reducing mitochondrial oxidative stress via CD38-mediated Sirt3 activation. Thus, CD38 inhibition may serve as a possible therapeutic strategy for the treatment of diabetic kidney disease.

