Are We Misunderstanding Aging? A New Look at Life-Extending Interventions
While current research explores a complex web of biological targets, a less mainstream framework suggests there may be only one that truly matters: mitochondria.
Highlights
- Mitochondrial dysfunction can be associated with all known fundamental drivers of aging.
- Mitochondrial dysfunction is also associated with most age-related diseases, including dementia, heart disease, and diabetes.
- Avoiding specific habits and taking certain supplements may prevent or alleviate mitochondrial dysfunction.
In 2013, scientists divided biological aging into nine processes known as the hallmarks of aging. For decades, researchers examined aging through the lens of these hallmarks, one of them being “mitochondrial dysfunction.” Then, after a decade of progress, three more hallmarks were added, totaling 12 aging hallmarks.
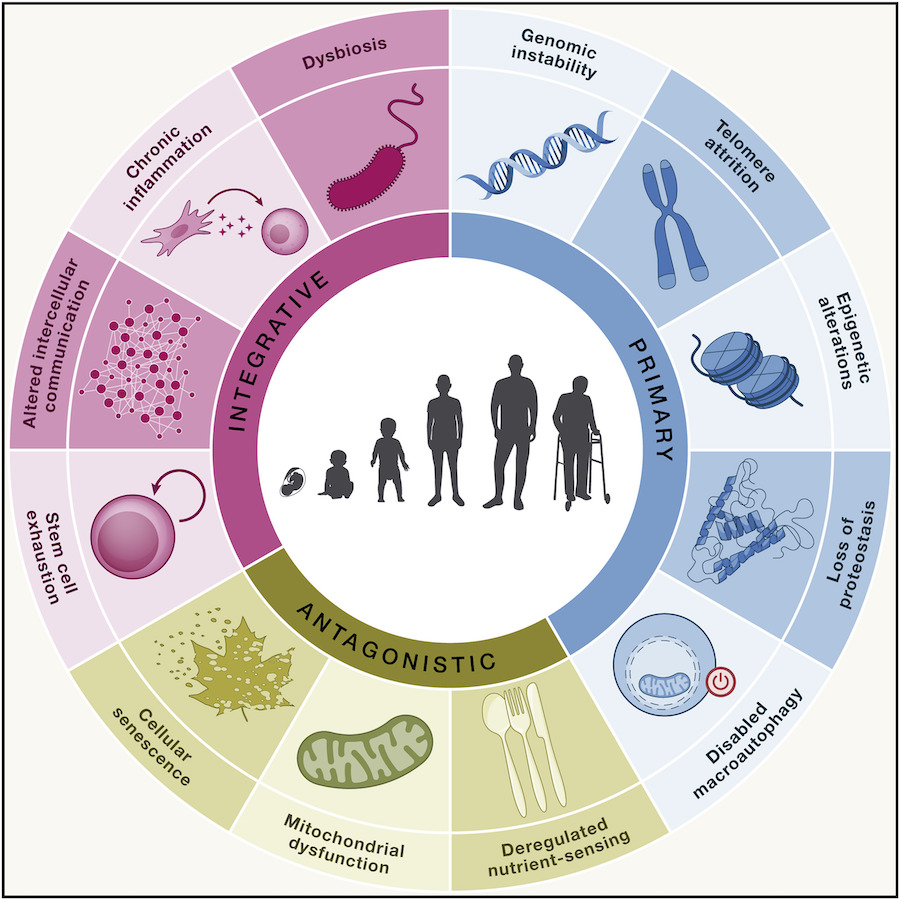
The hallmarks of aging provide a framework for researchers to develop life-extending interventions. However, not all scientists agree that the hallmarks of aging are equal. In fact, some scientists argue that “mitochondrial dysfunction” is the most important hallmark. That is, some researchers argue that dysfunctional mitochondria are the root cause of aging.
Mitochondrial Misconceptions: Unlearning Grade School Biology
While in school, many of us created models of animal cells. It was then that we learned mitochondria are the “powerhouses of the cell.” However, these models were usually not accurate, often depicting one oversized mitochondrion. Of course, it is difficult to create an accurate 3D model of a cell. The point was to introduce the basic anatomy of a cell.

Mitochondria is plural for mitochondrion. Some cells, namely heart cells, can have up to 8,000 mitochondria. This helps explain why the heart, the most metabolically active organ in the body, is composed of about 30% mitochondria. Moreover, the hundreds to thousands of mitochondria that reside in our cells are interconnected, constantly joining together (fusion) and breaking apart (fission) to maintain mitochondrial health.
Mitochondria Are Underrated
The primary function of mitochondria is to generate ATP (adenosine triphosphate). When the chemical bonds of ATP are broken, energy is released. This energy is utilized by our cells to function and survive. Illustrating the importance of ATP: Cyanide, a poison that shuts off ATP generation by inhibiting mitochondria, leads to death within minutes of ingestion.
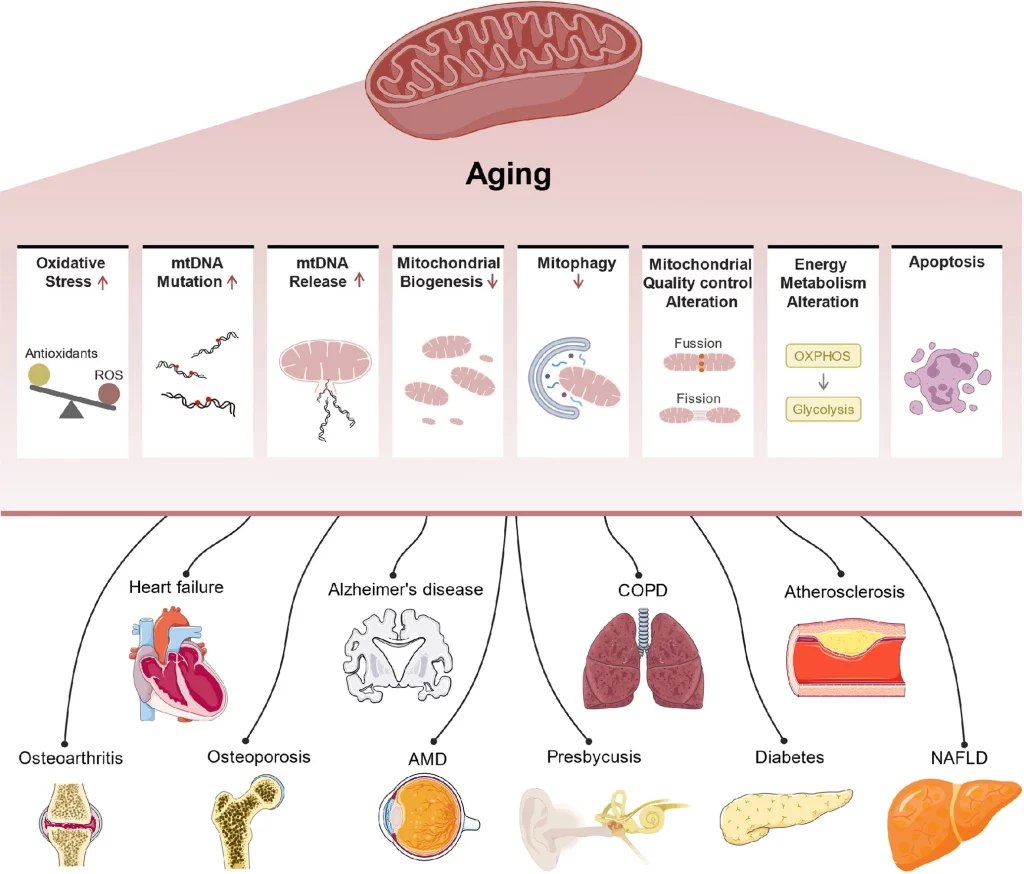
How Mitochondrial Dysfunction Triggers Fundamental Drivers of Aging
ATP is necessary for life, and the primary source of ATP is mitochondria. Could it be that the gradual dwindling of ATP, caused by mitochondrial dysfunction, is the root cause of aging? We explore this question through the lens of the hallmarks of aging, summarizing the work of Danish scientists from their recent publication in Aging Cell.
Through the process of generating ATP, mitochondria produce small amounts of highly reactive molecules known as reactive oxygen species (ROS). ROS are essential signaling molecules, and small quantities are not harmful. However, dysfunctional mitochondria produce excessive levels of ROS, causing oxidative stress, one of the hallmarks of aging.
Oxidative stress occurs when ROS damage cellular components like DNA, leading to two other hallmarks:
- Genomic instability: Accumulated DNA damage and mutations.
- Telomere attrition: The shortening of the protective sequences of DNA at the end of chromosomes.
DNA damage from oxidative stress can also cause our cells to enter a state of cellular senescence, a crucial hallmark of aging. Senescent cells can accumulate with age, promoting chronic inflammation, yet another hallmark of aging. Oxidative stress also promotes chronic inflammation by activating pro-inflammatory molecules.
The original nine hallmarks of aging in 2013 placed inflammation under another hallmark: intercellular communication, which refers to the communication between cells. It wasn’t until 2023 that chronic inflammation became its own hallmark. Along those lines, inflammation remains the most detrimental aspect of dysregulated intercellular communication in the context of aging.
Dysfunctional mitochondria, oxidative stress, and inflammation are among the multitude of factors contributing to the stem cell exhaustion aging hallmark. Stem cell exhaustion is the inability of our stem cells to regenerate tissues, making it one of the more important hallmarks when it comes to counteracting degenerative aging.
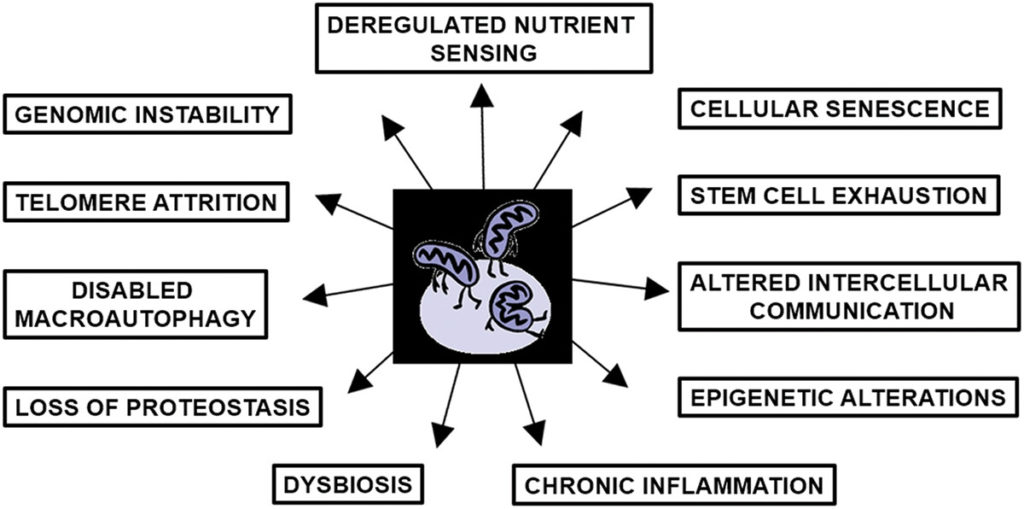
The relationship between mitochondrial dysfunction and the remaining hallmarks is less obvious. Although, through mechanisms like damaged mitochondrial DNA, there is evidence supporting the role of dysfunctional mitochondria in promoting deregulated nutrient sensing, dysbiosis, disabled macroautophagy, epigenetic alterations, and the loss of proteostasis.
Nevertheless, all these processes ultimately require ATP. If mitochondria are impaired, they generate less ATP. This brings up a crucial question: What actually causes mitochondrial dysfunction? The answer may lie in the preventative measures associated with counteracting mitochondrial dysfunction.
Preventing Mitochondrial Dysfunction
Mitochondrial dysfunction can potentially be prevented or alleviated by avoiding lifestyle habits like overeating and being sedentary.
Overeating
Overeating involves eating too much food (consuming excess calories), especially in one sitting. High-calorie macromolecules like fat, along with sugar refinement, allow for the production of calorically dense (high-calorie per volume) processed foods, making it easy to overeat. Moreover, processed foods often lack the nutrients necessary to efficiently metabolize food. This is why whole foods, such a fruits and vegetables, which are high in nutrients and low in calories per volume, are recommended.
Consuming fewer calories and/or fasting are the most powerful life-extending interventions, according to the abundance of scientific evidence. Through the lens of mitochondria, overeating overwhelms our mitochondria, causing them to generate excessive levels of ROS. Since they generate the ROS, mitochondria and mitochondrial DNA are in the closest proximity to ROS. This makes mitochondria highly susceptible to oxidative stress. As such, mitochondria are among the first cellular structures to be damaged by ROS, leading to mitochondrial dysfunction.
Being Sedentary
A lack of physical activity can reduce the number of mitochondria we have. A sedentary lifestyle reduces mitochondrial biogenesis, which is the production of new mitochondria. Especially when combined with overeating, a lack of mitochondria can promote oxidative stress. This is because the fewer mitochondria we have, the more likely they are to be overwhelmed by excessive caloric intake. Moreover, remaining sedentary leads to increased fat and reduced muscle mass. The less muscle we have, the fewer calories we burn at rest, making overrating particularly harmful.
Diet and exercise are among the best methods for maintaining normal mitochondrial function with age. Supplementing diet and exercise with certain dietary compounds may also potentially contribute to improving mitochondrial function.
Supplements
NAD+ (nicotinamide adenine dinucleotide) carries electrons to mitochondria. Mitochondria use these electrons to generate ATP. NAD+ is also used as fuel for enzymes called sirtuins, which contribute to maintaining mitochondrial function. Taking NAD+ precursors like nicotinamide, NR (nicotinamide riboside), or NMN (nicotinamide mononucleotide) can boost NAD+ levels, which studies show are low in certain individuals, such as older and obese individuals.
Urolithin A is a compound that has been shown to stimulate mitophagy, a process that maintains mitochondrial function. Mitophagy is thought to decline with age; thus, taking urolithin A may potentially counteract age-related mitochondrial decline. Combining urolithin A with NAD+ may provide synergistic effects in maintaining mitochondrial function. Longevity nutraceuticals, such as Restorin, already include compounds that boost NAD+ and promote mitophagy, making it ideal for targeting mitochondrial dysfunction.
What Causes Biological Aging?
The jury is still out on the precise causes of degenerative aging, but most scientists agree that the process is complex and multifactorial. Whether mitochondrial dysfunction is truly the root cause of aging remains an open question. However, mitochondrial dysfunction is one of the most crucial fundamental drivers of biological aging. Maintaining mitochondrial function helps to maintain overall health, thus increasing the probability of living longer without diseases. Therefore, avoiding overeating and routinely exercising are among the most powerful interventions that may potentially extend lifespan.

