Aubrey de Grey on the Future of Longevity and Aging Research
Aging expert Aubrey de Grey discussed how to make mice live longer and “making aging the new COVID” at Longevity Summit Dublin 2025.
Highlights
- To sidestep our ignorance about which specific types of physiological damage drive aging, Aubrey de Grey suggests combining multiple aging interventions that have some lifespan extension effects on their own.
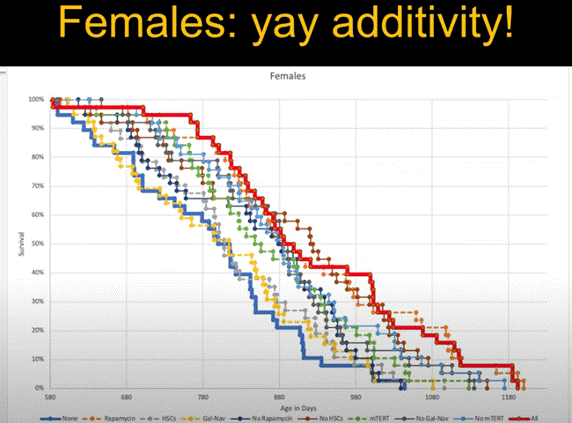
- Administering four aging interventions—mTERT, rapamycin, a senolytic, and hematopoietic stem cell transplantation—to middle-aged mice additively extended their lifespans more than any treatment individually.
- In the future, de Grey wants to treat middle-aged mice with a combination of eight other aging interventions in an attempt to stimulate further lifespan extension.
As discussed at the Longevity Summit Dublin 2025, in a study that cost roughly $3.5 million, aging aficionado Aubrey de Grey additively extended the lifespan of mice by combining four aging intervention treatments. When combined, the four aging interventions extended the lifespan of mice more than any intervention on its own. Aubrey de Grey conducted this experiment, which included some 1,000 mice, to address what he referred to as a slow rate of progress in tackling how to slow aging.
The aging interventions, which de Grey applied to middle-aged mice, were mouse telomerase reverse transcriptase (mTERT), rapamycin, an unidentified senolytic, and hematopoietic stem cell transplantation (HSCT), all treatments that have been shown to extend mouse lifespan on their own. mTERT is an enzyme that helps maintain the protective caps composed of DNA at the ends of chromosomes (telomeres) and helps counteract the age-related shortening of telomeres. Moreover, rapamycin is an immunosuppressant prescribed to prevent organ rejection in transplant recipients, which some researchers believe may promote longevity. Additionally, Aubrey de Grey used an unidentified senolytic, which selectively eliminates dysfunctional cells that accumulate with age, potentially rejuvenating tissues. Finally, HSCT involves infusing an aged organism’s bone marrow with younger stem cells to possibly prevent or delay aging.
Sidestepping What Researchers Do Not Know About Aging
Aubrey de Grey said that some researchers may think the slow progress made in aging over the last several years means that research should be geared more toward understanding aging processes better. In contrast, de Grey says he thinks that what we understand about aging is abundant enough to justify trying to find out what happens with the aging interventions already developed.
Along those lines, Aubrey suggested that we should sidestep what we do not know about the specific ways age-related physiological damage takes place. To achieve this, de Grey proposed a divide-and-conquer approach to intervene against aging. In that regard, Aubrey lamented that many aging interventions work partially, likely targeting certain aspects of age-related physiological damage. Moreover, combining these interventions could help to target multiple pathways of aging to extend lifespan greater than any of them on their own.
“The point is we gave [the interventions] in combination,” said Aubrey de Grey. “And the goal was to determine whether we could actually get a bigger benefit on lifespan in these mice than what you can get with any of these things individually.”
Combining Four Treatments to Additively Extend Lifespan
The results of the experiment were a little mixed, yet generally positive. For example, females treated with the four interventions showed a pretty clear additive lifespan extension, where combining the four interventions extended average lifespan better than any on its own. However, the results from males were a bit murkier. Although males did not exhibit as clean an average lifespan extension as females with the combination treatment, de Grey lamented that there was a general trend toward an additive effect.
Importantly, de Grey relayed that the individual intervention treatments on their own had some lifespan-extending effect. To him, this meant that each intervention had some influence on one or more aging pathways and that when combined, the interventions target an array of aging pathways to additively confer lifespan extension.
Aubrey de Grey’s Next $5 Million to $6 Million Experiment
In the future, de Grey wants to combine eight other aging interventions in an attempt to drive further lifespan extension. In that regard, he does not believe there is a maximum lifespan ceiling in mice. Rather, he thinks that combining more treatments can target multiple aspects of age-related damage to drive further lifespan extension.
The aging interventions that de Grey would like to try in his next experiment are deuterated fatty acids, inducible Yamanaka factors, primitive cell exosomes, pristine albumin, a new type of senolytic, antibodies that inhibit the inflammatory protein IL-11, inhibiting a protein called Cdc42, and acarbose. Aubrey’s proposed experiment with these eight aging interventions would cost between $5 million and $6 million and utilize some 2,000 mice.
Deuterated Fatty Acids
Deuterated fatty acids are the basic building blocks of fats in which some hydrogen atoms are replaced with deuterium (a heavier version, also known as an isotope, of hydrogen). The replacement of hydrogen atoms with deuterium creates more resilient fats that are less susceptible to damage from harmful molecules called free radicals. By making fats more resilient to damage, deuterated fatty acids protect cell membranes and potentially counteract age-related diseases arising from free radical damage, such as neurodegenerative diseases. While some research indicates that deuterated fatty acids may not significantly extend the overall lifespan of mice, they have been shown to improve cognition in a mouse model of Alzheimer’s disease.
Yamanaka Factors
Inducible Yamanaka factors encompass genetic material introduced into cells for the expression of three proteins (known as Yamanaka factors) that reprogram cells to a younger state. The Yamanaka factor proteins are used in a technique called partial epigenetic reprogramming. Epigenetics entails molecular tagging patterns on DNA that modify gene expression, and partial epigenetic reprogramming restores DNA tagging patterns toward those seen in more youthful states. Using this technique, researchers have been able to extend lifespan in mice.
Primitive Cell Exosomes
Primitive cell exosomes are tiny vesicles that carry proteins, fats, and genetic material derived from stem cells. These vesicles are believed to offer many of the regenerative benefits of stem cell transplantation without the potential side effects, such as fatigue and nausea, associated with transplanting whole, living stem cells. Research has shown that primitive cell exosomes extend the lifespan as well as improve physical performance and cognition in aged mice.
Pristine Albumin
Pristine albumin is a blood protein in a high-quality, unaltered form, and its pristine nature is crucial for maintaining the protein’s biological function. As such, albumin is the most abundant protein in human blood, with several important roles in the body, including maintaining fluid balance and protecting against inflammation. Using pristine albumin, which has not been exposed to physiological conditions that alter its structure or function, may work against oxidative damage, the harmful effects of damaging molecules called reactive oxygen species. Interestingly, research in mice has shown that regular injections of pristine albumin significantly extend mouse lifespan.
Senolytics
Senolytics are compounds that selectively eliminate cells that have reached a permanent state of growth arrest and sometimes release inflammatory factors called senescent cells. Using senolytics to lower the prevalence of senescent cells in tissues has been shown to rejuvenate multiple tissues and organs in mice. Furthermore, certain senolytics like fisetin have been shown to extend mouse lifespan.
Antibodies that Inhibit IL-11
Moving on, Aubrey has also proposed using antibodies that inhibit IL-11 proteins in his next experiment. IL-11 is a cytokine (small proteins that regulate immune responses) that promotes inflammation. The antibody inhibitors of IL-11 that de Grey proposes using are under development to treat liver disease and cardiac scarring (fibrosis). Intriguingly, IL-11-inhibiting antibodies have been shown to dramatically extend average lifespan in mice by 22.5% to 25%.
Cdc42 Inhibitors
Cdc42 inhibitors are compounds designed to block the activity of the protein Cdc42, which plays a crucial role in cell processes like signaling and migration. By interfering with Cdc42 activity, these inhibitors have shown potential to counteract diseases like cancer, where overactive Cdc42 contributes to tumor growth and spreading. Importantly, treating aged mice with the Cdc42 inhibitor CASIN significantly extended lifespan.
Acarbose
Finally, de Grey wants to test acarbose, a diabetes medication, in combination with the other seven aging interventions mentioned in his proposed mouse lifespan experiment. Acarbose helps control blood sugar levels by delaying the absorption of glucose into the bloodstream, especially after a meal. Notably, acarbose has been shown to extend average lifespan by 22% in male mice and 5% in female mice.
Making Aging the New COVID
Aside from planning his new experiment, Aubrey de Grey addressed why he has not moved on to performing clinical trials in humans. Along those lines, he said that he has not attempted to initiate these trials because he thinks there is too much apathy around aging research. In other words, de Grey wants more action from people around the world to address aging. He added that he wants people to care about aging research as much as they did about COVID research after the COVID pandemic hit in 2019.
“We just have to make aging the new COVID,” said Aubrey de Grey.
Aubrey also said that logic and information about aging, which he has tried to disseminate to the masses for some 20 years, have been more or less ineffective in promoting and garnering interest in aging research. To get around the lack of enthusiasm in aging research, de Grey recommends getting celebrities like Oprah Winfrey and influencers like Joe Rogan to present the public with information on longevity science more often. In that regard, he recalled that he and Harvard’s David Sinclair presented their research on Joe Rogan’s podcast a few times, which he believes has been useful publicity.
Getting Celebrities and Influencers Interested in Aging Research to Drive Publicity
To gain more attention from celebrities and influencers, Aubrey said that getting mice to live dramatically longer with combinations of treatments, as with his experiment, could win their enthusiasm. He also said that if more aging researchers dared to make predictions as to when we could reach a point where advancements in lifespan extension outpace life expectancy (a concept known as longevity escape velocity), we could make aging the new COVID very quickly.
A New Strategy to Drive Aging Research Toward Reaching Longevity Escape Velocity
Aubrey de Grey’s discussion of how to tackle aging in mice using a divide-and-conquer approach, which involves multiple interventions targeting different pathways of aging, could provide researchers with new ways to counteract aging. Not only that, but if this tactic dramatically extends mouse lifespan at some point, this news could capture the attention of more celebrities and influencers, who would then relay the information about the achievements in aging research to the public. In doing so, de Grey hopes that we can make aging the new COVID, where researchers fervently work to figure out how to address and potentially treat aspects of aging. Perhaps Aubrey’s newest strategy to move aging research along will serve as a way to propel the aging research field toward achieving longevity escape velocity in the next few decades.