Blood Plasma Exchange Slows Alzheimer’s in Landmark Clinical Study
The AMBAR (Alzheimer's Management by Albumin Replacement) study research group demonstrates that transplanting blood plasma with albumin protein improved memory, language, and quality of life in patients with mild-to-moderate Alzheimer’s.
Highlights
- Plasma exchange improved language fluency and processing speed in patients with mild Alzheimer’s disease.
- This treatment improved short-term verbal memory in patients with moderate Alzheimer’s disease.
- The quality of life improved, and the neurological status was stable in these Alzheimer’s patients.
One powerful anti-aging technique used on rodents in labs is the transplantation of blood from a young mouse to an old mouse. While this may feel a bit like a horror flick, blood plasma exchange may be the answer for people suffering from age-related conditions like the neurodegenerative disease Alzheimer’s.
The AMBAR (Alzheimer’s Management by Albumin Replacement) trial group reports that plasma exchange with albumin replacement affects neuropsychological, neuropsychiatric, and quality-of-life outcomes in mild-to-moderate Alzheimer’s disease patients. These findings confirm the potential of plasma exchange with albumin replacement as a new modality of treatment in Alzheimer’s disease.
What is plasma exchange with albumin replacement?
The blood plasma from Alzheimer’s patients’ contains the toxic neurodegenerating compound amyloid-beta (Aβ) bound to circulating albumin — a protein made by your liver that helps keep fluid in your bloodstream to avoid leaking into other tissues and also carries various substances throughout your body, including hormones, vitamins, and enzymes. Researchers have posited that routine plasma exchange of Alzheimer’s patient’s plasma, containing albumin-bound Aβ, might help clear the Aβ in the blood as well as in the cerebrospinal fluid (CSF) that soaks our brain.
The AMBAR process entails a multimodal approach to the management of Alzheimer’s. On the one hand, periodic plasma exchange eliminates beta-amyloid protein so that it does not accumulate in the brain and cause neuronal damage. On the other, the albumin administered may have other beneficial effects due to its antioxidant and anti-inflammatory properties, helping to slow the disease progression.

The AMBAR study
The trial, which was designed by Grifols in collaboration with Ace Alzheimer Center Barcelona and the Alzheimer’s Disease Research Center in Pittsburgh, Pa., U.S., aimed to evaluate the efficacy and safety of plasma exchange with albumin (combined with intravenous immunoglobulin in some treatment arms) in patients with mild and moderate Alzheimer’s disease. The AMBAR clinical trial was an international, multicenter, randomized, double-blind, with a parallel assignment and placebo-controlled study that enrolled patients with mild and moderate Alzheimer’s from 41 treatment centers in Spain and the United States.
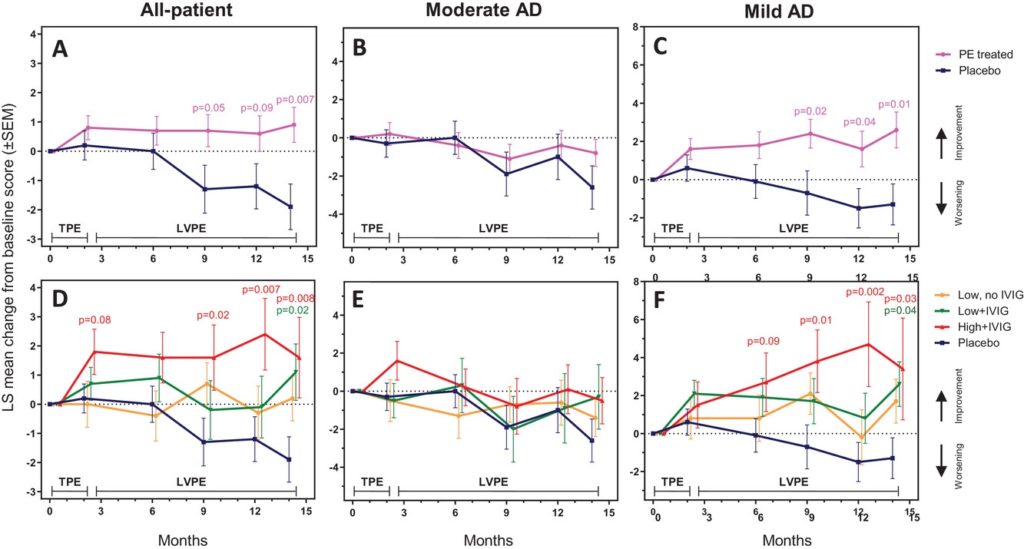
In 2020, the results of the primary endpoints of the AMBAR trial were reported, demonstrating that plasma exchange treatment could slow down the decline of cognitive, functional, and global assessments in Alzheimer’s disease. In the present study, the AMBAR trial research team present the results of the neuropsychological (including memory, language, and attention/executive functions), neuropsychiatric (including depression and suicide), and quality-of-life outcomes of the AMBAR study.
Plasma exchange with albumin replacement slowed down the decline of cognitive, functional, and global assessments in Alzheimer’s disease
Here the AMBAR study researchers observed that the plasma exchange treated patients, especially those with high levels of albumin replacement, were more frequently associated with statistically significant differences in the change from baseline. This was particularly evident in mild Alzheimer’s patients’ language fluency and processing speed tests.
Measures for quality of life are paramount in Alzheimer’s because of the devastating impact on both patients and caregivers. Effects on quality of life as measured by the caregiver were better in plasma exchange-treated than in the placebo arm, especially in the moderate Alzheimer’s patients. By contrast, the positive effects of plasma exchange on quality of life based on patients’ rating were seen at month 14, with mild Alzheimer’s patients reporting improvement in quality of life, but not those with moderate Alzheimer’s.
The plasma exchange approach did not negatively affect the neuropsychiatric status of patients. Moreover, the apparent reduction of neuropsychiatric manifestations and depression symptoms in mild Alzheimer’s patients could be attributable to the placebo effect since moderate Alzheimer’s patients may be less conscious of being administered treatment.
The battery of neuropsychological tests in the AMBAR study showed statistically significant improvement to placebo (i.e., effect size >100% in change from baseline scores) in specific Alzheimer’s severity subgroups and treatment arms, typically at more than one assessment time point. So, plasma exchange-treated patients showed the intellectual decline commonly associated with the progression of Alzheimer’s disease and could even be able to learn and recover. Moreover, the trend was observed in several cases as early as a peak in month 2, the end of the conventional and more intensive plasma exchange period.
When can plasma exchange with albumin replacement be used to treat Alzheimer’s?
Overall, the neuropsychological battery results suggest that Alzheimer’s patients could benefit from plasma exchange even at the early stages of Alzheimer’s. Because mild Alzheimer’s patients typically have less insoluble Aβ deposits in the brain, it is suggested that mechanisms beyond removal of albumin-bound Aβ may have been involved in the plasma exchange approach. For example, plasma exchange will need to be accompanied by treatments that eliminate dysfunctional albumin, inflammatory mediators, and other proteins, such as possible pro-aging factors.

