Could Activating a Longevity Gene Extend Our Lives?
Researchers in China report that activating a gene linked to human longevity—OSER1—extends lifespan in multiple animal models of aging.
Highlights
- Increasing OSER1 gene activity prolongs the lives of silkworms, nematode worms, and flies.
- Although the function of OSER1 remains largely unknown, researchers showed that it works against cell stress, which may lead to lifespan extension.
- Mutations in this gene have been linked to longer lifespans in humans.
Published in Nature Communications, Dai and colleagues from Southwest University in China showed that increasing the activation of a gene—OSER1—and concomitantly, increasing the protein it produces, significantly extends longevity in silkworms, nematode worms, and flies. What’s more, humans possess the OSER1 gene, and an analysis associated certain mutations in this gene with human longevity. These interesting findings may give us an idea of a potential target gene for gene therapy to possibly extend lifespan in the future.
“We identified this protein that can extend longevity. It is a novel pro-longevity factor, and it is a protein that exists in various animals, such as fruit flies, nematodes, silkworms, and in humans,” says Dr. Lene Juel Rasmussen, one of the authors behind the study in a press release.
An Age-Regulating Protein, FOXO, Activates the OSER1 Gene
The process of identifying OSER1 started with examining genes influenced by a protein that regulates aging and lifespan called FOXO. Since FOXO has been tied to aging regulation, Dai and colleagues had the idea that certain genes FOXO activates may play a role in longevity.
To find which genes FOXO activates, the researchers used silkworm cells. Increasing and decreasing FOXO expression in these cells helped pinpoint several silkworm genes that FOXO activates. For example, 3,185 out of a total of 14,623 genes showed increased activation associated with elevated FOXO. Further genetic analyses revealed that nine of the silkworm genes that FOXO binds to also exist in humans.
The researchers then manipulated the nine identified genes also present in humans—including OSER1—in nematode worms to measure effects on lifespan. Importantly, of these genes, reducing the activation of OSER1 shortened lifespan the most, by about 17%. With that finding, the China-based researchers honed further analyses on OSER1.
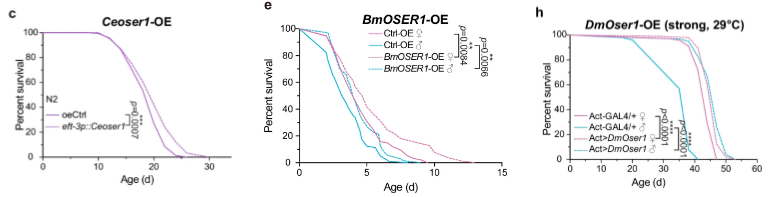
Additional analyses revealed that genetically manipulated silkworms, nematode worms, and flies with increased and decreased OSER1 activation exhibited prolonged and reduced lifespan, respectively. These results suggest that OSER1 strongly influences lifespan in multiple species, where increasing and decreasing its activity either extends or shortens lifespan, respectively.
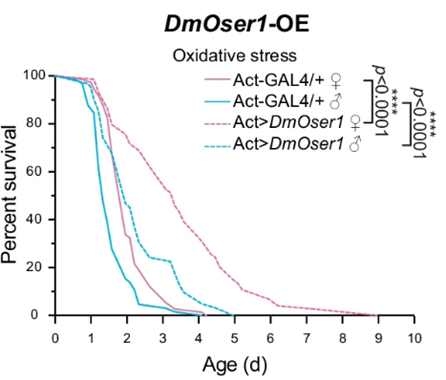
Since the FOXO protein stimulates enzymes that alleviate cell stress from harmful molecules called reactive oxygen species (ROS), Dai and colleagues sought to find whether the FOXO-activated gene, OSER1, also plays a role against ROS. To do so, they treated flies with a compound that triggers ROS formation and subsequent cellular stress called paraquat. As expected, flies exposed to cellular stress via paraquat lived shorter lives, but genetically engineered flies with higher OSER1 activation exposed to paraquat showed partially restored lifespan. These data suggest that increasing OSER1 activation may work, at least in part, by reducing cell stress from ROS to extend lifespan.
Dai and colleagues then analyzed human data to see whether an association exists between long-lived individuals (who lived at least 96 years) and the prevalence of certain mutations in OSER1. Interestingly, mutations in seven points of DNA (known as small nucleotide polymorphisms [SNPs]) in the OSER1 gene were associated with longer lifespans. This finding suggests that not only does OSER1 influence longevity in multiple animal species, but it may also do so in humans.
Developing a Lifespan-Promoting Drug or Gene Therapy Targeting OSER1 May Take Decades
The findings from Dai and colleagues’ study may hint that scientists could potentially develop therapies to target the OSER1 gene to extend human lifespan. Such a therapy could be as simple as a gene therapy that increases the mRNA in cells to enhance the production of the OSER1 protein. Of course, studies in organisms like mice and non-human primates would need to precede human clinical trials testing such a gene therapy for safety and efficacy confirmation. However, since not much is known regarding the function of the OSER1 gene and its protein, many things could go wrong along the lines of triggering cancer through increasing its abundance in multiple tissues throughout the body.
For this reason, before more testing is done to find out whether targeting OSER1 with gene therapy could extend human lifespan, more studies need to uncover what functions it has. This may also help to unravel whether increasing it in only certain tissues, such as the kidneys or liver, may have a more advantageous effect than increasing it pervasively in most tissues throughout the body.
What’s more, the possibility looms that a pharmaceutical drug could be developed that increases OSER1 gene activity. Since gene therapies can confer dangers, such as the aforementioned possibility of increasing the risk of cancer, a drug may serve as a better tool to enhance OSER1 activation.
Essentially, though, the discovery of OSER1 as a potential gene target for human lifespan extension only constitutes the tip of the iceberg. In that regard, performing the research necessary to create a gene therapy and/or pharmaceutical to increase its expression could take decades. Aside from that, key take-home messages from this discovery are that researchers continue to progress in their search for aging interventions and that a true means to counter aging may come from genetics research.
“We are currently focused on uncovering the role of OSER1 in humans, but the lack of existing literature presents a challenge, as very little has been published on this topic to date. This study is the first to demonstrate that OSER1 is a significant regulator of aging and longevity. In the future, we hope to provide insights into the specific age-related diseases and aging processes that OSER1 influences,” says Zhiquan Li, a contributing author to the study.

