Daily Vitamin D Supplementation Slows Cellular Aging: New Trial Finds
A new analysis from the VITAL study suggests daily vitamin D₃ supplementation may help slow cellular aging by reducing telomere shortening in white blood cells.
Highlights
- Daily supplementation with 2,000 IU of vitamin D₃ over four years modestly slowed immune cell telomere shortening in older adults, suggesting a potential role in reducing age-related cellular decline.
- The findings from the VITAL trial offer some of the strongest randomized clinical evidence to date that vitamin D₃ may support genomic stability and protect against telomere erosion.
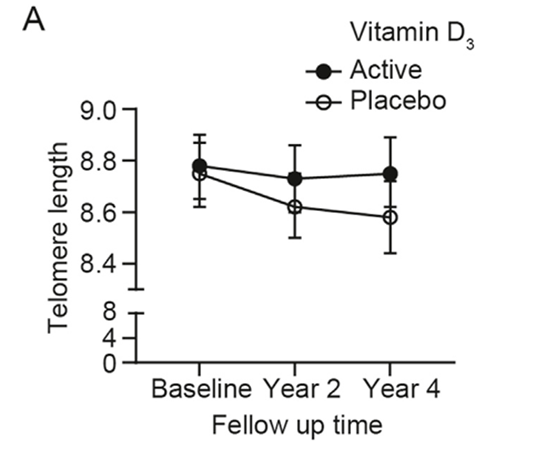
A new analysis from the Vitamin D and Omega-3 TriaL (VITAL), published in The American Journal of Clinical Nutrition, offers compelling evidence that daily vitamin D₃ supplementation may help slow cellular aging. Researchers found that four years of vitamin D₃ supplementation at 2,000 IU per day preserved approximately 140 base pairs (bp) of telomere length in leukocytes, white blood cells vital to immune function. This finding suggests a potential protective effect against age-related cellular deterioration.
In adults, telomeres – the protective caps of repetitive DNA at the ends of chromosomes – naturally shorten by about 25 to 27 base pairs per year. This study’s observed telomere preservation is therefore potentially equivalent to delaying cellular aging by nearly three years, reinforcing the idea that vitamin D may play a meaningful role in maintaining genomic stability as we age.
Why Telomeres Matter for Aging and Health
Telomeres function much like the plastic tips on shoelaces, preventing chromosomes from fraying or sticking to each other. As cells divide over time, telomeres gradually wear down. When they become too short, the cell either dies or enters a state called senescence, in which it stops dividing and begins secreting harmful inflammatory molecules. Telomere shortening is widely recognized as a hallmark of biological aging and has been associated with increased risk for chronic diseases such as cancer, cardiovascular disease, and type 2 diabetes. Maintaining telomere length is considered a promising target for promoting longevity and delaying the onset of age-related dysfunction.
The VITAL Study and Telomere Sub-Analysis
The VITAL study enrolled more than 25,000 adults across the United States to investigate the long-term effects of vitamin D₃ (2,000 IU/day) and marine omega-3 fatty acids (1 gram/day) on major health outcomes, including cancer and cardiovascular disease. Within this larger trial, a subgroup of 1,106 participants (mostly healthy older adults) was included in a telomere sub-study.
Participants were randomly assigned to one of four groups: vitamin D alone, omega-3 fatty acids alone, both supplements combined, or placebo. Blood samples were collected at the beginning of the study and again four years later to evaluate changes in leukocyte telomere length using quantitative polymerase chain reaction (qPCR), a standard method for assessing relative telomere length.
Vitamin D Preserves Telomeres Over Time, Omega-3s Do Not
The results showed that participants who received vitamin D₃ alone experienced significantly less telomere shortening than those given a placebo. On average, vitamin D preserved around 140 base pairs of telomere sequence over the four-year period. This effect remained statistically significant even after controlling for factors such as age, sex, race, and body mass index, indicating that the benefit was independent of these variables. The protective effect of vitamin D was also observed regardless of whether participants were simultaneously receiving omega-3 supplements.
In contrast, omega-3 fatty acid supplementation alone did not significantly influence telomere length. This suggests that while omega-3s may have beneficial effects on cardiovascular health and inflammation, they do not appear to directly impact telomere dynamics over the observed period. The finding adds to ongoing debates regarding the role of omega-3s in cellular aging and emphasizes the specificity of vitamin D’s potential in this context.
How Might Vitamin D Protect Telomeres?
Although no definitive mechanism has been established linking vitamin D to telomere preservation, emerging evidence suggests a potential relationship through its roles in immune modulation and genomic maintenance. Vitamin D₃, once converted to its active form, binds to the vitamin D receptor (VDR), which regulates genes involved in inflammation, oxidative stress, and DNA repair, all pathways that can impact telomere stability.
Epidemiological studies have supported this link: a large UK Biobank study of over 148,000 adults found that low vitamin D levels were associated with shorter leukocyte telomere length. Similarly, earlier work in the American Journal of Clinical Nutrition reported that women with the highest vitamin D levels had significantly longer telomeres, equivalent to roughly five years of reduced cellular aging, compared to those with lower levels. While these findings do not establish causality, they align with the VITAL trial’s observed attenuation of telomere shortening following vitamin D₃ supplementation.
Strengths, Limitations, and Future Research
This study is among the first large-scale, long-term randomized controlled trials to provide evidence that daily vitamin D supplementation may reduce the rate of telomere shortening in humans. The trial’s robust design, including its randomized assignment, long follow-up period, and large sample size, adds confidence to the results. However, some limitations remain.
The average effect size, while statistically significant, was modest, and it is still unclear whether this degree of telomere preservation will translate into reduced disease risk or extended lifespan. Additionally, since the study cohort consisted mostly of older adults with relatively sufficient baseline vitamin D levels, the findings may not be generalizable to younger individuals or those with severe deficiencies.
Looking ahead, further research is needed to determine whether higher doses of vitamin D or longer treatment durations could lead to more substantial benefits. It would also be valuable to investigate whether the combination of vitamin D with other lifestyle or pharmacological interventions could enhance its effects on telomere length and cellular aging. Studies that incorporate more advanced biomarkers, such as epigenetic clocks or direct markers of senescent cell accumulation, may help clarify vitamin D’s broader geroprotective potential.
Model: Healthy Older Adults
Dosage: Vitamin D 2,000 IU/day

