Diabetes Drug Henagliflozin Shows Signs of Slowing Biological Aging in Clinical Trial
Trial shows that henagliflozin, a diabetes drug, lengthened telomeres and improved aging biomarkers in patients with type 2 diabetes after 26 weeks of treatment.
Highlights
- People with type 2 diabetes who took henagliflozin daily for 26 weeks had longer telomeres in their white blood cells compared to those on placebo. Telomeres are protective caps on the ends of chromosomes that shorten as we age.
- The drug improved blood sugar control, reduced body weight, and raised IGFBP-3, a protein linked to healthier aging, while keeping its safety profile similar to that of a placebo.
Aging leaves clues scattered within the body before manifesting outwardly. Telomeres gradually shorten as cells divide, making them a widely studied marker of biological aging. Hormones such as insulin-like growth factor 1 (IGF-1) and its binding protein IGFBP-3 influence growth and repair, and their balance shifts with age. Immune cells also change over time, losing some of their ability to clear damaged cells. Studying these signals gives researchers a window into whether a therapy is affecting the biology of aging, beyond its usual medical role.
Putting Henagliflozin to the Test
Henagliflozin is a sodium–glucose cotransporter-2 (SGLT2) inhibitor, a class of diabetes drugs that helps the kidneys flush excess sugar out of the blood. Researchers launched a randomized, double-blind, placebo-controlled trial to see if it also altered signs of aging.
The study enrolled 150 adults with type 2 diabetes. Participants were randomly assigned to receive either 10 milligrams of henagliflozin or a placebo once a day for 26 weeks. By the end, 124 participants completed all primary assessments, giving researchers a clear look at how the drug affected key biomarkers.
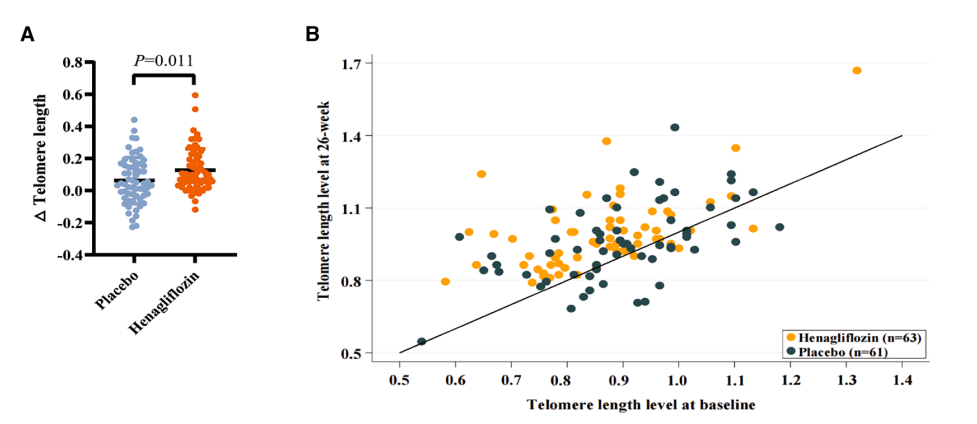
Telomeres Grew Longer
The standout finding was in telomeres. Blood tests showed that participants taking henagliflozin had longer telomeres in their white blood cells compared to those on placebo. Telomeres act like the plastic tips on shoelaces, keeping DNA strands from fraying. When they shorten too much, cells become less capable of dividing and repairing tissues. Preserving telomere length is considered one of the ways to extend cellular health.
Better Blood Sugar and Weight Control
Henagliflozin also improved classic measures of diabetes management. Participants on the drug saw larger drops in fasting blood sugar and hemoglobin A1c, a long-term marker of glucose control. They also lost more weight and had lower body mass index scores than those on placebo. These improvements reduce the strain that high blood sugar places on cells and may indirectly help slow aging-related damage.
A Shift in Growth Factor Signaling
Beyond metabolism, henagliflozin changed hormone pathways linked to aging. Levels of IGFBP-3 rose in the treatment group, shifting the balance away from strong IGF-1 signaling. IGF-1, or insulin-like growth factor 1, encourages growth and tissue building, but persistently high activity in this pathway has been tied to faster aging in animal studies. A higher ratio of IGFBP-3 to IGF-1 is generally thought to reflect a slower aging profile.
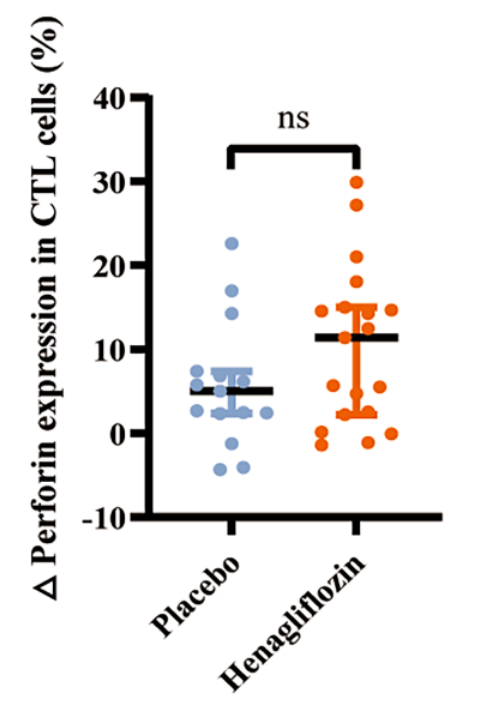
Stronger Immune Cell Activity
In a subset of participants, the drug appeared to sharpen immune function. Cytotoxic T cells, immune cells that patrol the body and remove damaged or infected cells, showed increased levels of proteins called granzyme B and perforin. These proteins are part of the toolkit T cells use to dismantle unhealthy cells. Keeping this arm of the immune system active may help maintain resilience as people age.
Metabolic Fingerprints of Fasting
Another shift came in energy use. Participants on henagliflozin had higher levels of β-hydroxybutyrate, a ketone that rises during fasting and has been linked to improved stress resistance in cells. Metabolomic profiling also showed increases in thiamine (vitamin B1), which supports energy production in mitochondria, and decreases in certain membrane lipids that can signal metabolic stress. Together, these changes suggested the body was operating in a state more similar to mild calorie restriction, a condition associated with healthier aging in many studies.
Safety Over Six Months
Henagliflozin was well tolerated. The overall rate of side effects was similar in both groups, and serious adverse events occurred only in the placebo arm. Researchers note that, as with all SGLT2 inhibitors, doctors should still monitor for rare issues such as genital infections or dehydration, but nothing new emerged in this trial.
Connecting Diabetes Care to Aging Biology
The trial suggests that henagliflozin can do more than lower blood sugar. Over six months, it influenced multiple hallmarks of aging, from chromosome protection to immune readiness and metabolic balance. The study was relatively short, so it cannot answer whether these changes will translate to long-term health or reduced disease risk. Larger and longer trials will be needed to explore that question.
Model: Human clinical trial — 150 adults with type 2 diabetes, randomized across multiple centers.
Dosage: Henagliflozin 10 mg orally once daily for 26 weeks.

