Testing if Exercise’s Effects Against Aging Can Be Transmitted
Aging researchers say it is time to initiate human trials testing blood plasma from people who exercise regularly against age-related conditions like cancer and metabolic disease.
Highlights
- Exercise-conditioned blood plasma comes from people who exercise regularly.
- Such exercise-conditioned blood plasma infused into older recipients may alleviate age-related conditions, including cancer, cardiovascular diseases, metabolic diseases, and neurodegeneration.
- Aging researchers review the preclinical data from rodent models, which support the possibility of exercise-conditioned blood plasma counteracting age-related physiological decline.
Goh and colleagues from the National University of Singapore published an editorial in the International Journal of Biomedical Sciences in which they proposed beginning human trials testing exercise-conditioned blood plasma—collected after multiple sessions of regular exercise—in aged recipients. Such human trials would evaluate exercise-conditioned blood plasma’s ability to alleviate age-related conditions, such as cancer, cardiovascular disease, metabolic disease, and neurodegeneration.
Muscle Contractions Induce the Release of Signaling Molecules that Promote Healthspan Extension
The rationale behind testing exercise-conditioned blood plasma comes from muscle contractions eliciting a complex array of molecular responses across organs following a round of exercise. These exercise-induced molecular signals persist after the exercise, and their long-term accumulation after multiple rounds of regular physical activity facilitates systemic adaptations. These adaptations extend beyond the musculature, resulting in organ system remodeling and perhaps an enhanced duration of life without a debilitating disease (a concept known as healthspan).
Accordingly, a bout of exercise mobilizes thousands of proteins, mRNA, and microscopic vesicles (extracellular vesicles), transporting them to distant sites throughout the body. These mobilized factors, referred to as exerkines, subsequently modulate organs, such as the brain, fat tissue, and the liver, in ways researchers are just beginning to uncover.
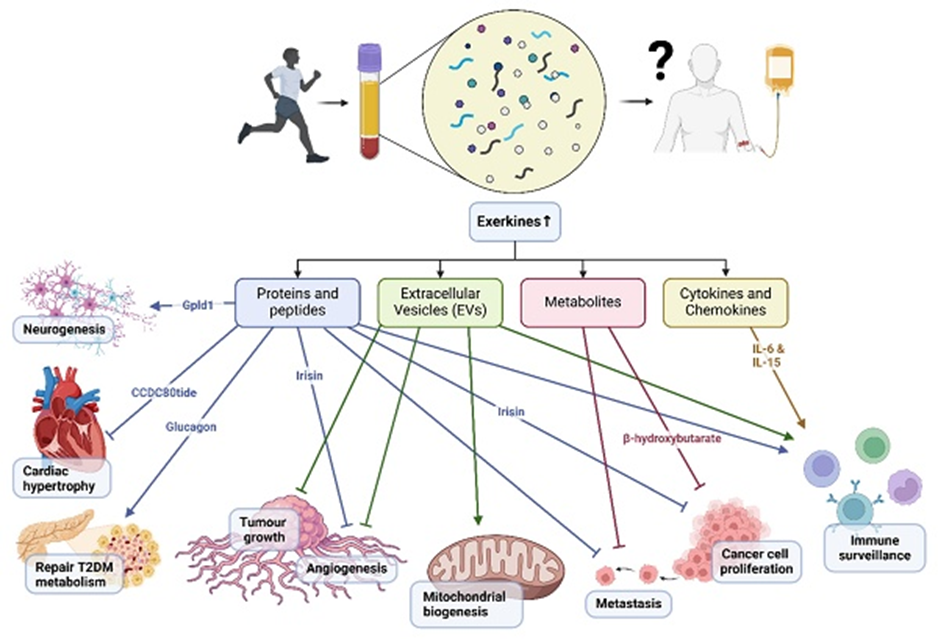
What Research Says About Exercise-Conditioned Plasma
Research has provided some evidence that the release of exerkines during muscle contractions is, in part, responsible for the multi-organ rejuvenation effects derived from exercise. Along those lines, a preclinical rodent study showed that transfused blood plasma from exercised rats improved neuronal viability and increased neuron generation (neurogenesis) three-fold in Alzheimer’s disease model rat recipients. Furthermore, researchers transfused blood plasma from young mice that underwent rigorous exercise into old mice, which significantly improved the proliferation of neurons in a memory-associated brain region (the hippocampus) in the old mice.
Moreover, an ongoing human study is exploring the transfusion of blood plasma from exercised individuals into patients with Alzheimer’s disease. In this study, researchers obtained blood plasma from young, well-trained, and aerobically fit individuals and transfused the blood plasma into patients with Alzheimer’s disease once every three months. After confirming the safety of this procedure, the study will subsequently evaluate whether the exercise-conditioned blood plasma from young donors improves things like cognition and quality of life in patients with Alzheimer’s disease. If this human trial yields positive results, this would lend support to the notion that exercise-conditioned blood plasma alleviates some features of neurodegeneration.
Exercise-Conditioned Plasma May Rejuvenate Aged People Who Are Bedridden or Paralyzed
Research has already demonstrated tissue rejuvenating effects of transfusing blood plasma from young rodent donors into older ones. Goh and colleagues’ proposal of using exercise-conditioned blood plasma adds a layer to this technique, increasing exerkines in young blood plasma via regular exercise.
This technique may be especially beneficial for aged patients who are bedridden or paralyzed and who may not have the ability to obtain all of the rejuvenating effects of regular physical activity. Along those lines, research suggests regular exercise may prevent or control some age-related diseases and increase life expectancy. As such, aged people who are bedridden or paralyzed may attain some of the molecular benefits of exercise through exercise-conditioned blood plasma without the need for physical exertion. Confirmation of this notion can only come from starting human trials with exercise-conditioned plasma, as Goh and colleagues proposed.

