FDA-Approved Antibiotic Extends Lifespan In Both Young and Old Lab Worms
Already known to have brain-protective and anti-inflammatory properties in mice and humans, minocycline extends lifespan up to almost 50% in a common lab animal used to study aging.
Highlights
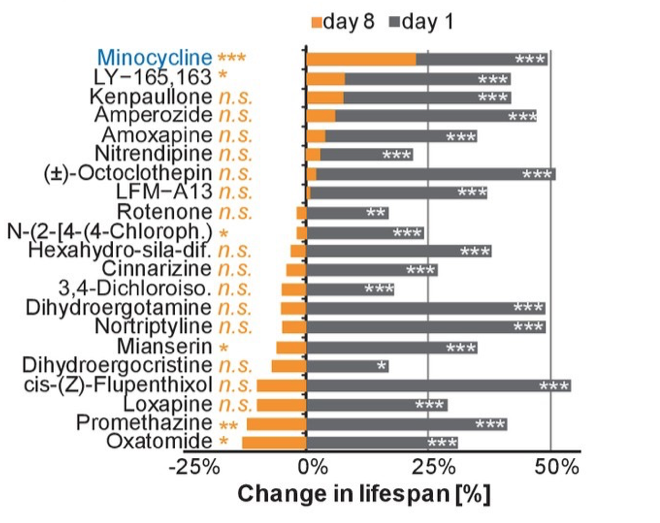
· Out of 21 compounds that increase lifespan in young C. elegans, only minocycline extended lifespan in worms treated starting in adulthood.
· Minocycline, which is already FDA-approved as an antibiotic for acne, reduces toxic protein aggregation even in old worms.
· The neuroprotective and aggregation-preventing effects of minocycline are explained by its attenuation of protein synthesis.
Our ability to deal with stress and clear out toxic protein aggregates decreases with aging and lies at the root of some neurodegenerative diseases like Alzheimer’s and Parkinson’s disease. Ideally, treatment would consist of using a drug to block or reduce toxic protein aggregates and extending life- and healthspan in older organisms at the first appearance of neurodegenerative symptoms or biomarkers.
Researchers from The Scripps Research Institute and The Buck Institute for Research on Aging showed in Elife that the antibiotic minocycline increases lifespan and reduces protein aggregation in the worm C. elegans. Of note, out of 21 compounds that extended longevity in young worms, only minocycline could increase C. elegans longevity at later stages of adulthood when the signaling pathways that manage the stress induced by toxic protein aggregation are deactivated. These results show that this antibiotic, which is already an FDA-approved compound for treating acne and has shown brain-protective effects in mice, lowers the concentration of newly synthesized aggregation-prone proteins, which lie at the core of many neurodegenerative diseases.
“Our studies on minocycline shed light on the plasticity of longevity mechanisms upon aging and reveal a mechanism of action for minocycline that explains its geroprotective effects,” said Solis and colleagues.
Targeting Stress Resistance and Protein Balance in Aging and Longevity
Aging is multifaceted, and age-related diseases come in many different forms. Yet, at the core of these phenomena are several pathways, including stress resistance and maintaining healthy protein levels (proteostasis). For example, though we don’t know what triggers neurodegenerative diseases like Alzheimer’s and Parkinson’s, there is compelling evidence for an age-associated collapse of proteostasis that contributes to the protein aggregation seen driving the deterioration of the nervous system. Others have shown that as animals age, their ability to respond to stress dramatically declines.
Some researchers think it would be ideal for enhancing stress resistance and proteostasis to extend lifespan and healthspan by treating older organisms at the first appearance of neurodegenerative symptoms or disease biomarkers, such as protein aggregates. However, we know little about what distinguishes timing-specific longevity mechanisms from longevity mechanisms that remain responsive throughout life. Indeed, most longevity mechanisms do not extend lifespan in C. elegans — an important animal model for aging and longevity — when initiated past day 5 of adulthood.
Minocycline extends C. elegans lifespan in both young and old animals
Ameliorating pathological protein aggregation requires pharmacological mechanisms that improve proteostasis, even when stress signaling pathways are compromised, as is the case with aging. To identify such mechanisms, Solis and colleagues searched for a compound capable of extending lifespan and improving proteostasis in post-stress-responsive C. elegans.
To this end, Solis and colleagues treated 8-day-old adult worms adults with 21 different molecules previously identified to extend lifespan. When treatment began on day 1 of C. elegans adulthood, all 21 molecules extended lifespan. But out of these 21 compounds, only minocycline extended lifespan when treatment was initiated 8 days into adulthood, which is considered a stage in which C. elegans adults are post-stress-responsive. Minocycline still extended lifespan when treatment was initiated on day 8, albeit by 22% instead of the 48% observed when treatment commenced on day 1.
Minocycline attenuates protein aggregation in both young and old C. elegans
Solis and colleagues next tested minocycline for its ability to modulate protein aggregation, a common molecular characteristic of many neurodegenerative diseases. The researchers treated the worms that express the human version of α-synuclein — a protein that forms aggregates central to the development of Parkinson’s — fused to a fluorescent molecule in the muscle with water or minocycline on day 1 of adulthood and imaged them on days 8, 11, 16, and 19.
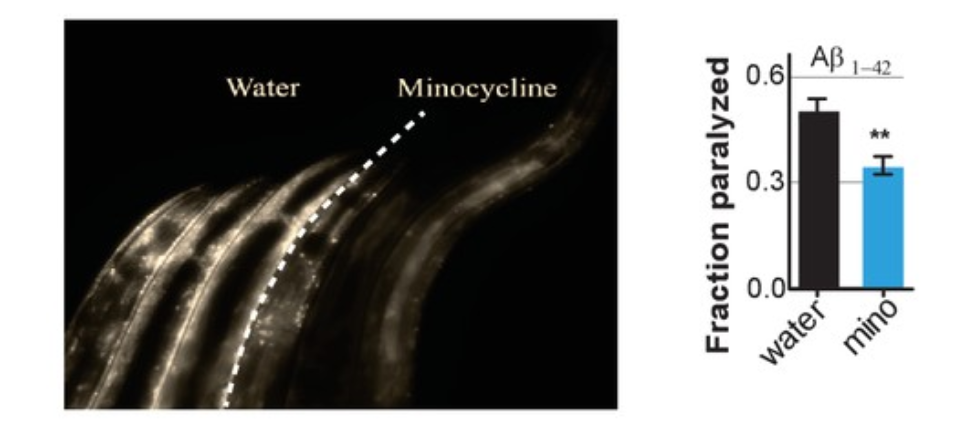
Minocycline suppressed the age-dependent increase in α-synuclein aggregation. Also, the antibiotic reduced α-synuclein aggregation even when added at a late age (day 8), when C. elegans no longer induced the stress response. To confirm whether this effect extended beyond α-synuclein aggregation to other neurodegenerative diseases, Solis and colleagues repeated these experiments in a model of Alzheimer’s diseases where the aggregation of proteins — in this case beta-amyloid (Aβ1-42) — paralyzes the worms and found that minocycline inhibited this paralysis. So, these experiments show that minocycline treatment reduced both age-dependent and temperature-induced protein aggregation in C. elegans.
Minocycline attenuates mRNA translation in yeast, worms, and human cells
Through a series of experiments in single-celled organisms (yeast), multi-celled organisms (worms), and human cells, Solis and colleagues then tested for minocycline’s mechanism of action. They find that the way minocycline works is by reducing protein synthesis (translation) independently of the stress response while mimicking the beneficial effects of this pathway. Reducing the concentration of newly synthesized aggregation-prone proteins relieves demands on the proteostasis network even in older individuals in which stress signaling pathways and protein degradation pathways have already been compromised due to advanced age.
These results also suggest that the neuroprotective and aggregation-preventing effects of minocycline, observed in preclinical mouse models and human clinical trials, can be explained by its attenuation of translation. While it is not known whether minocycline extends lifespan in mammals, its effects reduce age-associated protein aggregation and inflammation, as evidenced by numerous preclinical and clinical studies.
Repurposing FDA-approved drugs like minocycline using screens reveals promising effects outside the primary indication — in this case, an antibiotic — and inevitably leads to promising new drug targets and mechanisms of action. Solis and colleagues say that this study suggests that in old individuals with incapacitated stress signaling pathways or autophagic pathways, pharmacological attenuation of cytoplasmic translation is a promising strategy to reduce protein aggregation. Altogether, it provides a lifespan-extending mechanism for the many beneficial effects of minocycline and related compounds called tetracyclines in models of neurodegenerative disease.

