Fisetin May Revitalize the Vascular System, According to New Study
Fisetin selectively eliminates dysfunctional cells in the vasculature that often produce inflammatory molecules to thereby enhance vascular function in old mice.
Highlights
- Dysfunctional vascular cells that often emit inflammatory molecules accumulate with age, and fisetin treatment selectively eliminates them.
- Researchers have identified a specific protein, CXCL12, emitted from these dysfunctional proteins, which enters circulation and partially mediates vascular dysfunction.
- In addition to eliminating dysfunctional vascular cells, fisetin treatment lowers CXCL12 levels in circulation and restores vascular function in aged mice.
Heart disease is the leading cause of death worldwide. Moreover, advancing age is the primary risk factor for heart disease due, in part, to progressive dysfunction of vascular cells. Cellular senescence—a state of permanent cellular growth arrest often associated with the release of inflammatory molecules— in certain vascular cells (known as endothelial cells) contributes to age-related vascular impairment. Moreover, previous research has shown that supplementing with senolytic agents, compounds that selectively eliminate senescent cells, improves vascular function in rodents, but details on how these compounds do so have not been fully elucidated.
Now, as published in a non-peer-reviewed preprint, Clayton and colleagues from the University of Colorado confirm that endothelial cells are highly susceptible to senescence in aged mice. Additionally, the researchers show that the senolytic agent, fisetin, selectively clears senescent endothelial cells. Furthermore, the research team found that an inflammatory factor secreted from senescent cells, called CXCL12, partially mediates vascular dysfunction. Additionally, fisetin reverses the elevated gene expression of CXCL12, lowers circulating levels of CXCL12, and helps restore vascular function.
This study confirms that fisetin improves vascular function and elucidates how it does so, in part by eliminating senescent endothelial cells and lowering circulating CXCL12. These findings also offer a new senescence-associated protein to target with future therapeutics, CXCL12, to improve vascular function with aging and possibly counteract heart disease.
Background on the SASP
Collectively, the inflammation-promoting factors released from senescent cells are referred to as the senescence-associated secretory phenotype (SASP). The release of the SASP inflammatory factors from senescent cells is believed to contribute to aging and disease. Not only that, but the SASP can trigger senescence in cells and tissue surrounding it, thus perpetuating senescence, especially as people age and their immune systems lose the capacity to effectively clear senescent cells.
As such, it is important to identify circulating SASP factors from senescent endothelial cells that significantly contribute to vascular dysfunction. The identification of such SASP factors can help researchers figure out how the SASP contributes to vascular disease and identify possible therapeutics that counteract these specific SASP factors’ effects.
Fisetin Selectively Eliminates Senescent Cells in the Vasculature and Lowers Levels of the SASP Factor CXCL12
For their study, Clayton and colleagues sought to examine the effects of supplementation with the senolytic agent, fisetin, on vascular function in aged and young mice. To do this, they treated old (27-month-old) and young (three-month-old) mice with oral doses of fisetin, given in an intermittent schedule each day for weeks one and five during a five-week treatment course.
To determine gene expression changes with age in the vasculature and which cellular pathways aging affects most, Clayton and colleagues ran a gene activity analysis on cells from the aorta, the largest artery in the body, after the five-week treatment period. They found that old mice treated with fisetin had a gene activity profile more similar to young mice than non-treated old mice.
Clayton and colleagues then ran an analysis to find which pathways were altered during aging, as shown in endothelial cells from old mice compared to young mice, which were also restored with fisetin treatment. Interestingly, the pathways they identified were involved in the SASP and inflammatory signaling. Collectively, these results demonstrate a gene activity shift in the vasculature with age and suggest that treatment with fisetin in old mice returns gene activity patterns toward a more youthful profile in the aorta, influencing the SASP and inflammatory signaling pathways.
Because the SASP represents a collection of pro-inflammatory molecules from senescent cells that modulate physiological function, which may, in part, initiate vascular dysfunction and heart disease, the researchers next sought to identify what specific SASP proteins facilitate vascular dysfunction. To do this, they measured which SASP proteins changed in abundance during old age and whether fisetin treatment reverses the change. Among the proteins, CXCL12 stood out because its levels significantly increased with age and because fisetin treatment lowered its levels in aged mice.
Clayton and colleagues then aimed to evaluate how fisetin and CXCL12 influence vascular function. To do this, they tested the effects of blood plasma from fisetin-treated old mice and also blood plasma with high levels of CXCL12, pumped through an artery removed from a mouse. Upon pumping the blood plasma through the artery, the researchers measured the artery’s dilation capacity, a key aspect of vascular function. Intriguingly, pumping blood plasma from fisetin-treated old mice through the artery conferred 14% higher dilation than that from non-treated old mice, suggesting that fisetin improves vascular function with advanced age. However, adding CXCL12 to blood plasma from fisetin-treated old mice reduced this dilation by 22%. These data suggest that the CXCL12 protein from the SASP factors facilitates, in part, vascular dysfunction, but that fisetin treatment can reverse it to some degree with advanced age.
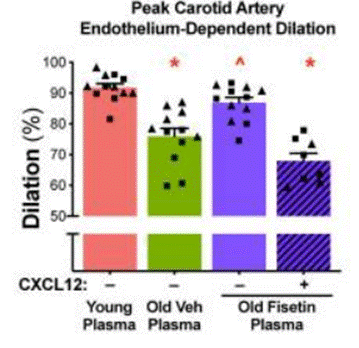
Since vascular dysfunction is believed to arise, in part, from the induction of cellular senescence, and because CXCL12 was shown to facilitate vascular dysfunction, Clayton and colleagues sought to determine whether elevated levels of CXCL12 induce endothelial cell senescence. To test this, the researchers exposed arteries to blood plasma from young mice, non-treated old mice, fisetin-treated old mice, and fisetin-treated old mice with high CXCL12 proteins added. Upon measuring a senescence marker in endothelial cells of the arteries, the researchers noted that fisetin alleviated the elevated abundance of senescent endothelial cells in old mice. However, increasing CXCL12 levels in the blood plasma from old mice treated with fisetin significantly increased the abundance of senescent endothelial cells. These findings thus added further evidence that fisetin lowers senescent cell abundance with advanced age and that CXCL12 mediates, in part, the induction of endothelial cell senescence.
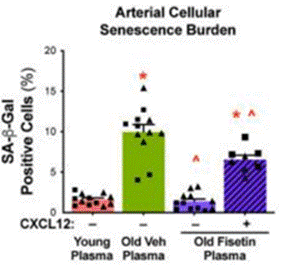
“In the present study, we assessed vascular cellular senescence at single-cell resolution in the context of aging and senolytic treatment with fisetin,” said Clayton and colleagues in their publication. “Our findings demonstrate that endothelial cells are particularly susceptible to undergoing cellular senescence with aging and that fisetin, a naturally occurring senolytic compound, effectively eliminates senescent endothelial cells in old animals.”
Human Trials Are Necessary to Confirm Fisetin’s Capabilities to Counteract Age-Related Heart Conditions
Clayton and colleagues’ study adds confirmation that supplementation with the senolytic agent, fisetin, improves vascular function. Fisetin’s enhancement of vascular function also arises through its capabilities to selectively eliminate senescent endothelial cells, which lowers levels of the SASP protein CXCL12.
Importantly, the study additionally identified CXCL12 as a key mediator in the SASP’s influence on vascular dysfunction. In that regard, scientists could tailor future therapies to specifically target CXCL12 to see whether doing so helps alleviate age-related vascular dysfunction and heart problems.
Moreover, since the study found that fisetin supplementation improves vascular function in mice, clinical trials should be done to determine whether these results translate to humans. Currently, 33 clinical trials are in the works testing fisetin, yet none of them apply to heart diseases. This means that future human trials testing whether fisetin improves vascular function and age-related heart conditions in humans by selectively eliminating senescent endothelial cells could shed light on whether fisetin is, in fact, a go-to senolytic with important aging intervention properties that apply to heart conditions.
Model: Three-month-old and 27-month-old C57BL/6JN mice
Dosage: 100 mg/kg/day of fisetin given by oral gavage daily during weeks 1 and 5 of an intermittent, 5-week-long schedule

