French Study Shows Senolytics Promote Survival from Aggressive Brain Tumors
Senescent age-associated cells worsen survival probability in glioblastoma cancer patients, yet removing senescent cells significantly increases the lifespan of mice with glioblastoma.
Highlights:
- Senescent cells are present in the tumors of patients with glioblastoma — brain and spinal cord cancer of cells called glia.
- Removing senescent cells with the senolytic (a compound that eliminates senescent cells) ABT263 increases the lifespan of a mouse model for glioblastoma by about 30%.
- Senescent cells are now associated with worse overall survival for glioblastoma patients.
Glioblastomas account for 15% of all brain tumors, affecting adults aged 45 to 70. Glioblastomas are the most aggressive — quickly spreading — brain tumor, but are largely treatment resistant due to difficulties in eradicating all cancerous cells. Considering the median survival time for glioblastoma is 15 months, treatments are in dire need.
“There is an urgent need to better understand the biology of the tumor, including the diversity of cell types of which it is composed, and their role,” explains Dr. Isabelle Le Roux, the senior author of a new study from Sorbonne University in Paris. ”The challenge is to find new therapeutic targets and significantly increase the lifespan of patients.”
Now, Le Roux and her team report in Nature Communications that senescent cells — cells that accumulate with aging and may drive aging itself — contribute to glioblastomas. Salam and colleagues show that multiple senescent cell markers are present in the tumors of glioblastoma patients and in a glioblastoma mouse model. Moreover, treating the glioblastoma model with ABT263 increases survival. Furthermore, senescent cells appear to contribute to poor survival outcomes in glioblastoma patients.
Senescent Brain Cells Reduce Lifespan
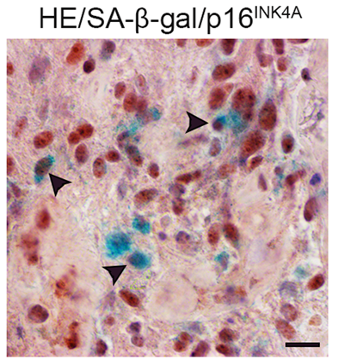
Salam and colleagues examined glioma tumors from 28 patients and observed several markers of senescence. These senescent cell markers were also observed in the glioma tumors of a mouse model for glioblastoma. This mouse model was generated by inducing mutations in key genes associated with glioblastoma. Overall, these findings demonstrate that senescent cells are present in human glioma tumors, which is recapitulated by the mouse model.
“Long considered a simple marker of aging, we now know that senescence occurs throughout life, especially in response to genotoxic stress – that is, an event that disrupts or damages DNA, such as chemotherapy,” says Alexa Saliou, the co-first author of the study.
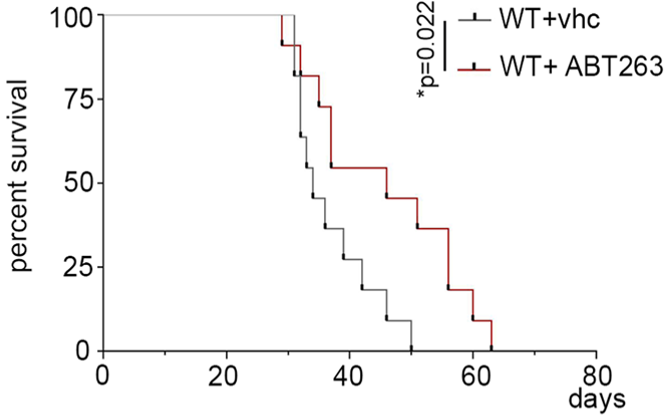
As with glioblastoma patients, the survival outcomes for mice with glioblastoma are poor. To determine if senescent cells contribute to survival, Salam and colleagues genetically removed senescent cells from mice modeling glioblastoma. They found that this increased the survival of the mice, suggesting that senescent cells contribute to poor survival outcomes.
In a similar experiment, the researchers fed the glioblastoma mice 50 mg/kg/day of ABT263 for five days. ABT263, also known as navitoclax, is an anti-cancer drug that is commonly used as a senolytic — a compound that selectively destroys senescent cells. They found that ABT263 increased the median lifespan of the glioblastoma mice by nearly 30%, further suggesting that senescent cells contribute to poor survival outcomes, which can then be mitigated by drug intervention.
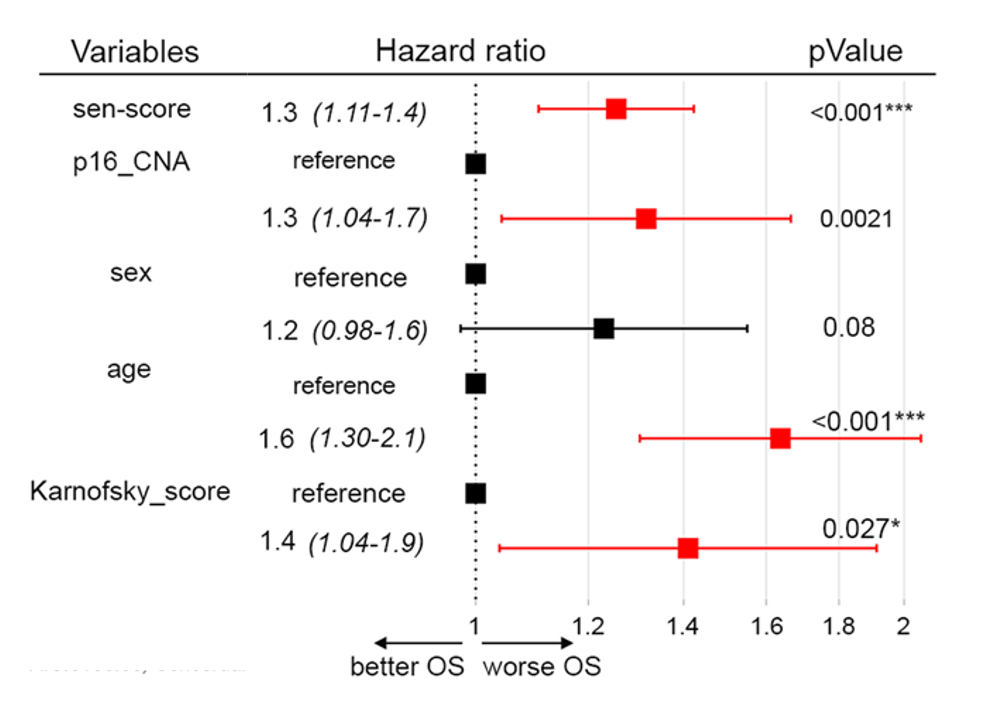
To evaluate how senescent cells could contribute to the survival of humans with glioblastoma, Salam and colleagues established a “senescent score” based on the 31 most highly activated genes they observed in glioma tumor senescent cells. With data from The Cancer Genome Atlas (TCGA), which contains information from glioblastoma patients, a survival analysis was conducted.
The survival analysis took into account five variables, including the senescent score, age, and sex. The Karnofsky score and p16 copy number alteration (CNA) were also included. The p16 CNA is based on the gene that codes for the senescent marker p16, which some cancer cells do not harbor. The Karnosfky score is based on a scale that measures how well cancer patients perform on daily tasks. The score ranges from 0 to 100, with 100 considered normal and 10 being terminally ill.
The survival analysis showed that, regardless of age, sex, p16 CNA, and Karonoskfky score, the senescent score predicted worse survival (hazard ratio above 1) in patients with glioblastoma.
“We observed that the strong expression of this [senescent cell] signature was associated with a poor prognosis,” adds Alexa Saliou. ”This shows the pro-tumor action of senescence in glioblastoma.”
Anti-Aging Molecules and Countering Cancer
Previous studies have shown that anti-aging molecules like nicotinamide riboside and niacin, which boosts NAD+, can aid in combating glioblastomas. Now Salam and colleagues show that senolytics, a class of drugs considered to be anti-aging molecules, could also aid in the treatment of glioblastomas. Still, the French researchers say a lot more work needs to be done to overcome several barriers. For example, ABT263 leads to side effects like low platelets and white blood cell counts. Another barrier is the blood-brain barrier:
“Eventually, we could consider treating patients with senolytics, i.e., molecules that target senescent cells to destroy them. In the near future, we hope to see the emergence of new senolytics capable of crossing the blood-brain barrier – which separates the brain from the general bloodstream. This is the big challenge today, as few therapeutic molecules are able to enter the brain. They will also need to cause few side effects if they are to be integrated into patients’ treatments. There is still a long way to go!”
Model: Glioblastoma mouse model
Dosage (oral): 50 mg/kg/day of ABT263 for 5 days

