How Do I Find Out My Biological Age?
A growing number of accessible and affordable at-home tests and walk-in clinics estimate your biological age by quantifying and combining an array of measurements from molecules to memory.
Life is finite, and we literally or figuratively blow out some candles demarcating our chronological age every living year. But as we get older, some become frail and require assistance in daily routines, and others remain independent and seem to escape significant physiological deterioration until very extreme ages. This varying limit to our existence has led many of us to wonder how much time we have left to live, and some of us have sought to figure out when we will die. In doing so, we’ve referred to palm readers and crystal balls to reveal to us the exact moment of the day we will cease to live.
Nowadays, you can pay to get your biological age — or how old you really are — estimated based on measurements from just a sample of saliva or blood or by taking an online quiz. While these tests won’t offer the minute or hour, let alone day, you will die, just spitting into a tube and sticking it in an envelope or getting your blood drawn at a clinic to running on a treadmill while being hooked up with a mask and wires can give you a pulse on your biological age. Just do an online search for “biological age test,” and you’ll find a seemingly endless list of options that measure all sorts of stuff, from patterns of molecules to your mental and physical abilities.
So, what exactly are all these biological tests measuring, and why do we measure them?
Biological age and biomarkers of aging
The functionality of our cells, tissues, and bodies — or biological age — is not perfectly tied to the chronological clock that ticks at the same rate for everyone. Although our chronological age can be somewhat linked to the onset of chronic diseases, biological age appears to be a far better predictor for specific health conditions, longevity, and mortality. This has led research institutions and biotech companies to search for the best way to measure biological aging.
According to the American Federation for Aging Research, a biomarker of aging should predict the rate of aging and monitor basic processes that underlie aging. An aging biomarker also needs to be repeatedly testable without harming the test subject and work in both humans and laboratory animals. While we’ve flirted with various concepts, the challenge of establishing a valid biomarker of aging is still ongoing.
Nonetheless, landmark papers have outlined aging’s key biological hallmarks (or pillars), serving as a springboard for many candidate biomarkers for biological age. Since aging is complex, aging biomarkers can theoretically be derived from any level of the organism — from molecules and cellular processes to physiological frailty and function — at which measurable age-related changes occur. That said, most biological aging tests performed through a mail at-home kit or a network of participating clinicians and labs are based on the molecular side of the spectrum.
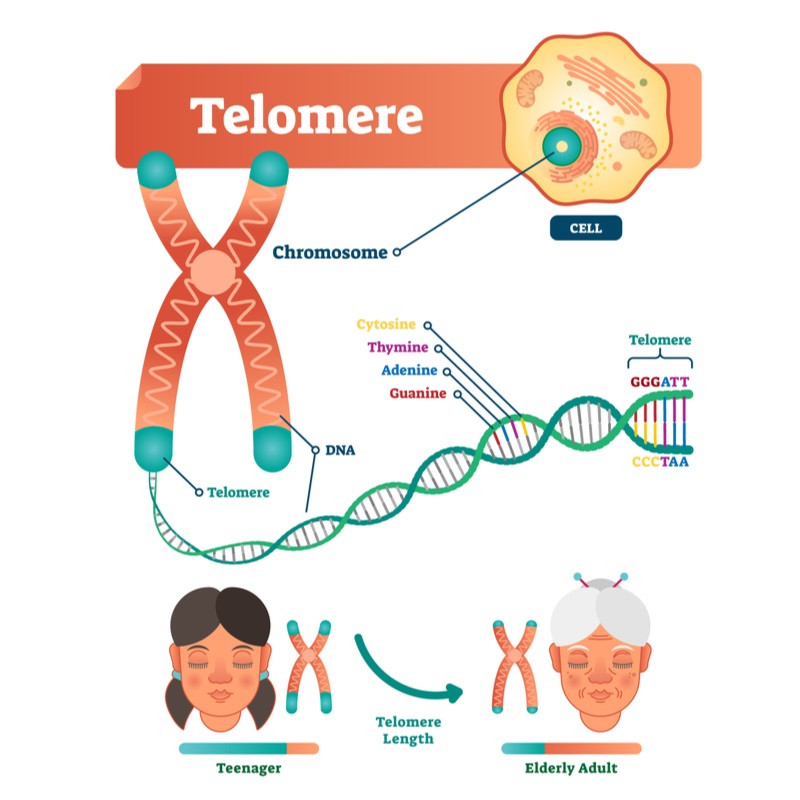
Telomere Length
In every cell, at the ends of DNA strands sit protective caps called telomeres. These structures shield the DNA that encodes the blueprint to life from sticking and fraying when cells replicate.
Telomeres are an interesting aging biomarker for several reasons:
- Inherited defects in the function of the enzyme critical to maintaining telomere length (telomerase) are associated with age-associated human diseases, including many cancers, diabetes, cardiovascular disease, and dementia.
- Telomere length shortens with chronological age and predicts the onset of cellular senescence — an age-related state of cell growth arrest.
- Telomere length and telomerase activity are heritable in both humans and mice, suggesting that it could account for some genetic variation seen in the aging process.
These insights have created interest in assessing the link between telomere length and biological aging. For example, researchers tied leukocyte telomere length to higher mortality in an analysis of a large cohort of people in the general population.
Interestingly, in the most extensive available study of telomere length in people to date, once people hit 75, age became positively associated with telomere length. In other words, those with longer telomeres at age 75 lived longer than those with short telomeres. However, not all studies have been able to reproduce the association with mortality. Yet, even though an analysis of many studies on telomere length associations with mortality confirmed the association between telomere length and all-cause mortality, there was a lot of inconsistency between studies.
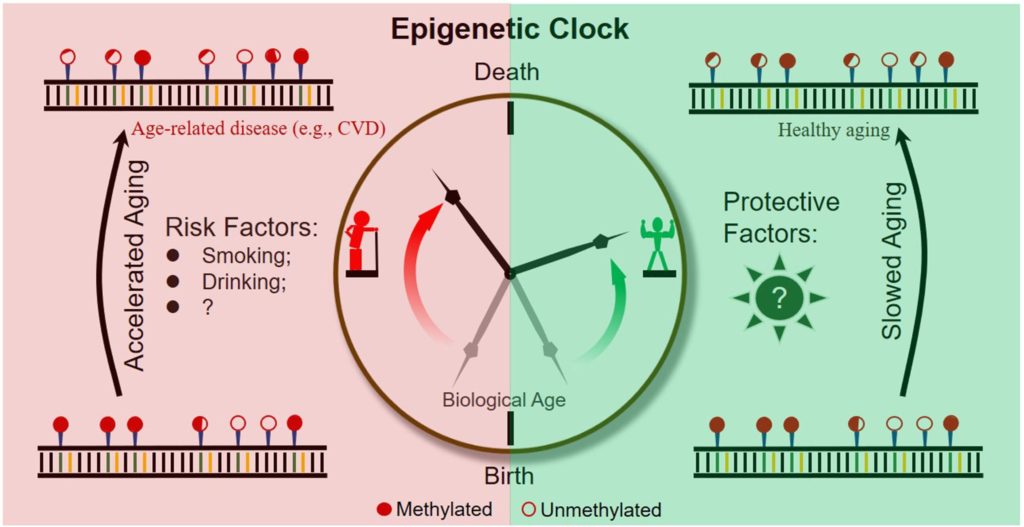
Epigenetic Clocks
Epigenetics is the study of how gene activity gets regulated. Epigenetic changes involve modifications to proteins and DNA that change gene activity without modifying the DNA sequence. Our cells can create the many different types of cells that populate the human body, all from the same set of DNA and react to environmental changes through epigenetics.
By tracking the levels and patterns of epigenetic modifications that correspond to age, researchers have developed “epigenetic clocks.” How epigenetic clocks estimate chronological age can be obscure; this is not wholly unexpected, given their derivation from computer learning algorithms — sometimes called artificial intelligence (AI) — sorting through patterns of epigenetic modifications.
You may be asking yourself, how do these patterns of epigenetic modifications relate to aging? Some researchers think that epigenetic modifications and maintenance systems change as a response to damage to DNA.
The most common type of epigenetic modification may be what’s known as DNA methylation. This epigenetic modification is influenced by genetics and the environment. Offspring of supercentenarians (105–109 years old) demonstrate DNA methylation patterns consistent with lower “epigenetic age” than do offspring of non-supercentenarians at the same age. Likewise, social and behavioral factors associated with longevity — high fish intake, moderate alcohol consumption, high education — directly correlate with epigenetic age.
The first epigenetic clock could reliably estimate chronological age and chronological-age independent mortality risk. However, it couldn’t account for common disease risk factors. A later DNA methylation biomarker grounded in a composite of clinical markers of biological age yielded enhanced predictions of mortality and age-related disease. Even so, it was less predictive than the clinical markers used to create the model.
What really made epigenetic clocks tick accurately was when they were combined with clinical chemistry biomarkers. With this combo, researchers could predict all-cause and cause-specific mortality better than chronological age. A major advantage of this method is identifying healthy individuals at risk and younger ages before the onset of clinically measurable age-related deficits.
“Omics”-Based Biomarkers
Omics refers to a field of study in life sciences that focuses on large-scale data to understand life. For example, metabolomics studies the metabolic responses of living systems, and proteomics offers a comprehensive, quantitative description of an organism’s sum of protein levels. The patterns of proteins change as humans advance from neonates to adults.
Transcriptomics is the study of an organism’s full range of RNA molecules — the products of gene activity that encode for proteins — that exist at a given time. This collection of RNA molecules, or transcriptome, is highly dynamic, responsive to environmental changes, and influenced by our genetics and epigenetics. So far, studies have shown that patterns of RNA molecules track with other hallmarks of aging, such as epigenetics. Although transcriptomic age has been shown to correlate well with epigenetic age, it does not correlate that well with chronological age. Notably, the combination of these transcriptional and epigenetic markers led to synergism so that the combination outperformed either marker alone.
Physiological and blood-based biomarkers
Historically, the first estimates of biological age were based on blood-based markers that could be measured in the clinic and on functional tests. Blood-based biomarkers have to do with blood contents, ranging from the quantities of certain compounds, such as hemoglobin or cholesterol, to markers of inflammation and glucose resistance to the types of blood cells. Clinical measurements, which can be thought of as physical examinations, can include anything from body-mass index and waist circumference to blood pressure and heart rate to cognitive function. Such markers have a direct clinical interpretation, but even if they predict mortality better than chronological age, it is unclear how much they measure biological aging itself rather than health deterioration for other reasons.
Frailty Index
One of the best characterized measures of biological age is the frailty (or deficit) index. This composite index is based on the proportion of health deficits accumulated by individuals among a set of health items surveyed throughout the body. The health items (variables) include various signs, symptoms, laboratory measurements, disabilities, and diseases. A frailty index calculated from about 20 to 100 health variables gives reliable and comparable results.
The frailty index has been used in many epidemiological and clinical studies to grade the degree of risk of several adverse outcomes, including mortality, health service use, hospital-acquired complications, worsening health, and loss of independence. The frailty index can also infer the risk for health conditions like cognitive impairment, heart disease, and osteoporosis.
So, what’s the best way to measure biological aging?
Many studies have put these aging biomarkers head-to-head to see which performs best at predicting mortality. In one study that looked at several aging biomarkers, frailty index and one of the methylation clocks were the best predictors of mortality, while telomere length was the worst.
An essential point in the context discussed is that, since aging is a highly complex phenomenon involving multiple pathways and operating at various levels of the biological organization of a living system, it can hardly be accurately measured with a single biomarker. Different measurements of biological age apparently quantify various aspects of the aging process. Therefore, estimates of biological age obtained with varying approaches of measuring may not coincide with each other.
Indeed, each measure included in the composite score would likely point to different aspects of the aging process, such as the developmental program (DNA methylation), replicative history of a cellular lineage (telomere length), environmental stress (blood profile), etc. Various measures can complement each other, thereby improving the predictive power of the composite measure.
Nevertheless, biological age is dynamic, and the cost of routinely performing sophisticated biomarker-based calculations of biological age is prohibitive. Even so, it remains that any marker of age, regardless of accuracy, must be seen in the context of each person’s current and past psychosocial environments.