How Do NAD+ Precursors Get Incorporated Into Cells?
Precursors like nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR) take slightly different paths into and inside cells to boost NAD+ levels.
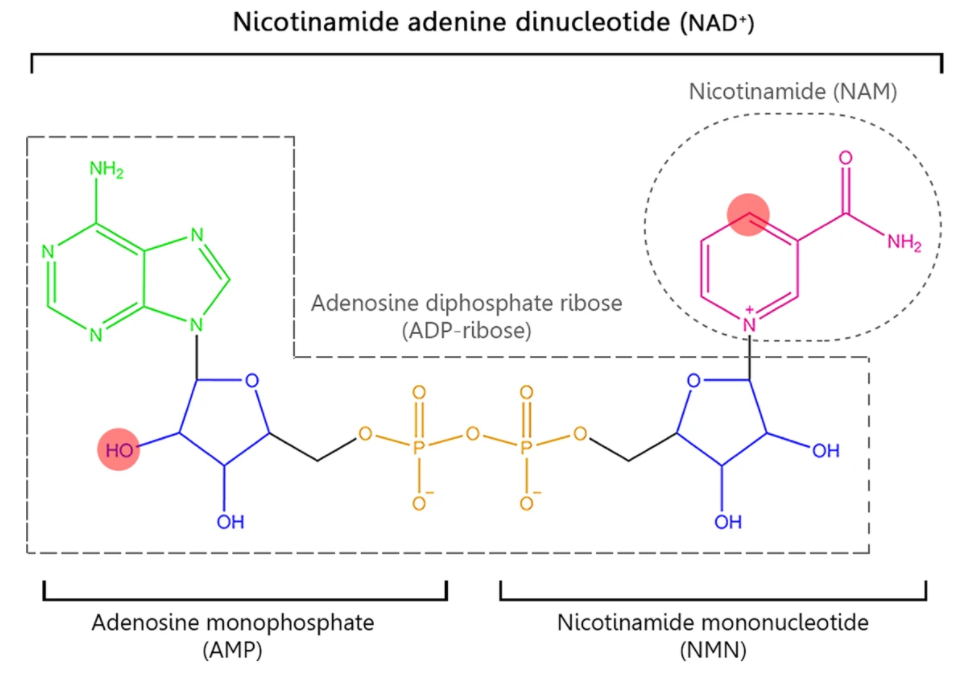
Various molecules, all differing by a few atoms, get passed along and transformed by different receptors and enzymes inside and outside cells to create the vital molecule nicotinamide adenine dinucleotide (NAD+). But these distinct NAD+ precursors, whose differences in atomic structures can be distinguished by just a letter in their formula or a word in their chemical name, are not all created equal.
Research shows that drinking water or eating food laden with these metabolic intermediaries or through supplements, whether consumed or injected, even if they all participate in the same pathway, do not have the same effect on NAD+ levels and cell, tissue, and body health and function. Part of their required dose and effectiveness may depend on how these NAD+ precursors get transformed by different enzymes and whether they can get into cells.
NAD+ Precursors and the Salvage Pathway
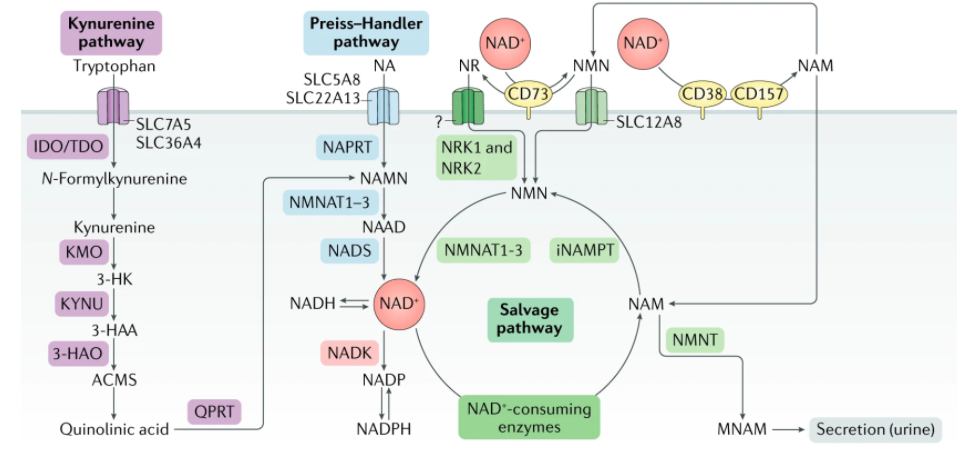
There are several pathways by which NAD+ can be generated. Still, one stands out called the salvage pathway because it is the most abundant in mammalian cells (the others are called the Preiss-Handler pathway, in which NAD+ is synthesized from nicotinic acid, and the de novo synthesis pathways, which starts from tryptophan).
The salvage pathway works based on the concept that NAD+ is constantly turning over; in essence, it is an NAD+ recycling pathway. When NAD+ gets consumed by enzymes, often participating in physiologic processes including DNA repair, metabolism, and cell death, it gets converted into nicotinamide (NAM) as a byproduct.
There are only two steps in the salvage pathway. The conversion of NAM primarily determines the rate of NAD+ synthesis in this pathway to nicotinamide mononucleotide (NMN) in this first step. Then, NMN is converted to NAD+ in the second step. All in all, to sustain NAD+ levels, NAM can be recycled back to NAD+ via the salvage pathway by being converted to NMN.
In addition to NAM, NMN can be generated by another NAD+ precursor called nicotinamide riboside (NR). Instead of being recycled from NAM emanating from NAD+ consumption, NR gets converted into NMN inside the cell by an enzyme family called nicotinamide riboside kinases (NRKs).
NMN and NR Have Different Cell Transport Mechanisms
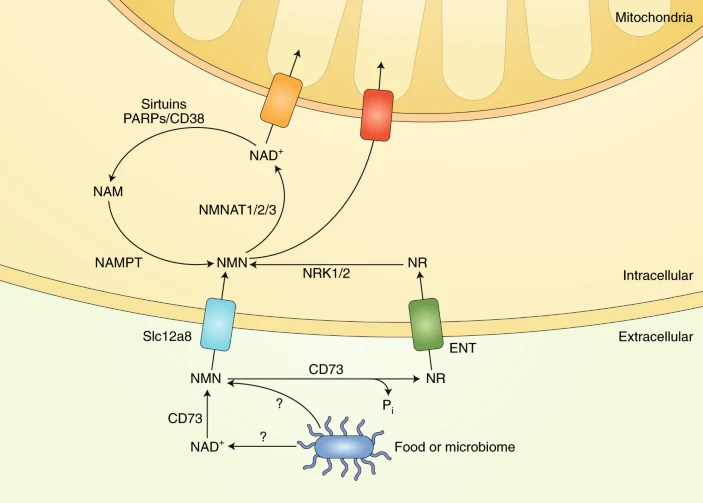
To make NAD+, the cell ultimately needs to get its hands on NMN. There are at least two ways that cells can do this: (1) by taking up NR that is converted to NMN inside of the cell or (2) taking up NMN directly.
NR is incorporated into cells using non-specific channels called equilibrative nucleoside transporters (ENTs). Studies have shown that members of the ENT family-proteins ENT1, ENT2, and ENT4 can import extracellular NR and a broad range of similar compounds into cultured human cells. ENT1 and ENT2 are found in most cell types and tissues, and ENT4 is located in the brain and heart.
A recent study identified a specific transporter of NMN called Slc12a8. Interestingly, Slc12a8 is found in great amounts in the small intestine, suggesting it is a means to incorporate NMN consumed through food or supplementation. What’s more, in this study, Slc12a8 levels increased in the small intestines of aged mice in response to a decrease of NAD+, suggesting that NMN supplementation is a viable way to boost NAD+ levels with age.
Also, it has been shown that some NMN gets converted outside of cells into NR by an enzyme called CD73, and this NR can then get converted back to NMN by NRKs within the cell. That being said, results clearly demonstrate unequivocal NMN uptake without any conversion of NMN to nicotinamide riboside outside of cells.
De Novo Synthesis of NAD+
Additionally, some cells, mostly in the liver, can synthesize NAD+ de novo from multiple dietary sources. NAD+ can be made de novo (latin for “from scratch”) from L-tryptophan via the kynurenine pathway or from vitamin precursors, such as nicotinic acid (NA), via the Preiss-Handler pathway.
Notably, the relative contribution of the de novo synthesis pathway to NAD+ levels is still not well understood. Outside the liver, most cells do not express the full array of enzymes necessary to convert tryptophan to NAD+ by the kynurenine pathway. Most tryptophan is metabolized to NAM in the liver, where it is released into the serum, taken up by peripheral cells, and converted to NAD+ by the NAM salvage pathway.
Additionally, under some circumstances, immune cells, such as macrophages, also make NAD+ from tryptophan. Thus, besides the liver, the de novo biosynthetic pathway seems to be a more indirect mechanism contributing to system-wide NAD+ levels, with most NAD+ coming from the NAM salvage pathway.
Aging and NAD+ Metabolism
NAD+ is constantly synthesized, consumed, and recycled in the cell to maintain stable cell levels of NAD+. However, during aging, this balance between the processes for synthesis and consumption of NAD+ can shift, and NAD+ degradation can outpace the ability of cells to make NAD+ de novo or their ability to recycle or salvage NAM effectively. Furthermore, excess NAM may be consumed via alternative metabolic pathways, effectively diverting it away from the NAM salvage pathway and further impacting NAD+ levels.
Also, key enzymes that consume NAD+ called sirtuins, PARPs, and NAD+ glycohydrolases have distinct roles in aging and age-related diseases. While enhancing the activation of sirtuins has emerged to increase lifespan and healthspan, aberrant activation of PARPs and NAD+ glycohydrolases, such as CD38, may exert the opposite effect exacerbate aging.
Therapeutic targeting of NAD+ level decline via supplementation
The good news is that the evidence for precursors like NMN and NR to boost NAD+ levels in humans is growing. Several human clinical trials are ongoing to assess the safety and efficacy of NAD+ augmentation. Importantly, early-phase trials of short-term NAD+ precursor administration have proven safe and increase NAD+ levels in healthy participants.
However, despite the promising preliminary results, whether long-term supplementation with NAD+ precursors has any side effects is still unknown. Moreover, many other questions need to be answered to deepen our understanding of the potential of NAD+-boosting therapies: is there any tissue/disease specificity for NMN and NR? What are the therapeutic doses of NAD+ precursors necessary for different diseases?
Nevertheless, using NAD+ precursors such as NMN and NR offers an exciting therapeutic approach to treat aging-related diseases and increase human healthspan. This is particularly important as elderly populations are rising rapidly, and aging-related conditions are predicted to cause a great deal of societal and economic burden in the coming decades. Hopefully, the upcoming results of current clinical trials will shed some light on our unsolved questions and set the basis for future directions in deciphering the role of NAD+ during aging in humans.