Chinese Study Shows Bone Formation Requires NMN
Research from Sichuan University shows that NAD+ is indispensable for stem cells from bone marrow to repair fractures.
Highlights
- In humans, NMN is necessary for bone stem cells to mature into bone-producing cells called osteoblasts.
- Enhancing NMN synthesis boosts bone stem cell maturation, produces more osteoblasts, and promotes bone formation.
- Blocking NMN synthesis impairs bone fracture repair in mice by inhibiting the development of bone stem cells into bone-producing cells.
When we break a bone, stem cells in our marrow choreograph a multistage process to repair and heal the fracture. These stem cells in the marrow are called bone mesenchymal stem cells (BMSCs) and are essential to bone regeneration. Delayed or failed bone fracture healing, which happens in 5-10% of cases, can often be traced back to deficient or dysfunctional BMSC activity.
Researchers from Sichuan University in China report that elevated levels of NAD+ are indispensable for BMSCs to produce bone-producing cells called osteoblasts and form bone. In mammals, NAD+ is predominantly synthesized from NMN by an enzyme called NAMPT. Li and colleagues show that blocking NAMPT stops human BMSCs from maturing into osteoblasts and reduces bone formation. On the other hand, elevating NAD+ levels by enhancing NAMPT activity increases BMSC maturation into osteoblasts and stimulates bone formation. This paradigm applies to cells in a dish and animals since blocking NAMPT in live mice inhibits bone fracture repair. Published in Stem Cell Research & Therapy, this study shows that NAD+ might provide a potential therapeutic target for bone repair and regeneration.
Building Bones Back Better
BMSCs are currently being exploited as seed cells for tissue regeneration and stem cell therapy. These self-renewable cells have the potential to differentiate into multiple types of cells, including osteoblasts and fat-producing cells (adipocytes). BMSCs transform into these cells in a mutually exclusive manner — that is, they typically mature into just one of these two lineages. Understanding how to tip the scales to control BMSC maturation towards osteoblasts has major consequences for bone repair.
A variety of factors contribute to the lineage commitment of BMSCs toward bone or fat formation, including extracellular environment and cell metabolism. The major pathway by which cells generate energy, oxidative phosphorylation, is one of the mitochondria’s most critical metabolic activities. Cells can also make energy in the cytoplasm through glycolysis — a less efficient means of generating energy than oxidative phosphorylation; the metabolism of sugars through mitochondrial oxidative phosphorylation can produce fifteen times more energy than glycolysis. But not much is known about the role of oxidative phosphorylation and glycolysis in regulating BMSCs cell fate decision and maturation.
Human Bone Formation Depends on NAD+ Production via NMN
Li and colleagues aimed to tease apart the roles of metabolism and NAD+ during BMSCs lineage commitment. The Sichuan University researchers show that cultured human BMSCs undergoing maturation into bone-producing cells shift their metabolism from glycolysis to oxidative phosphorylation. Consistent with enhanced oxidative phosphorylation, mitochondria become slender and increase in number dramatically during bone formation (osteogenesis). Conversely, BMSCs committed to maturing into fat-producing cells exhibit an overall increase in metabolism with increased oxidative phosphorylation and glycolysis activity.
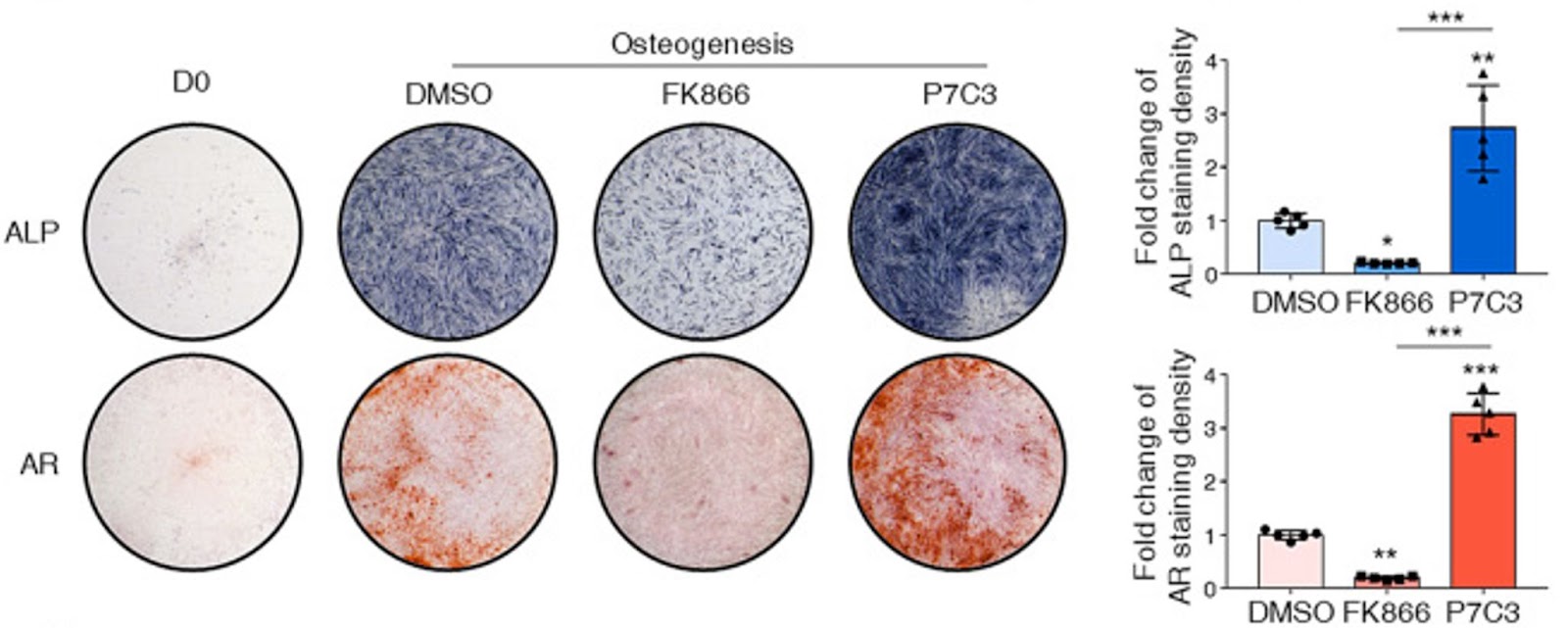
(Li et al., 2022 | Stem Cell Research & Therapy) Enhancing NAMPT activity increases bone generated from adult stem cells. On the one hand, enhancement of NAMPT via P7C3 promoted human BMSCs to become osteoblasts and form bone (osteogenesis). On the other hand, inhibiting NAMPT via FK866 suppressed the osteogenesis of human BMSCs and abolished the formation of mineralized nodules as stained by alizarin red. ALP (alkaline phosphatase) and AR (alizarin red) stain for bone formation in blue and red, respectively.
They also found that NAD+ was key for BMSCs to pick a bone-producing fate. When Li and colleagues reduced NAD+ level by repressing NAD+ synthesis with a NAMPT inhibitor (FK866), they observed impaired osteoblast maturation and bone production. These effects were reversed when NAD+ levels were elevated by the NAMPT activator (P7C3). Moreover, this study indicates that the NAD+ is necessary to sustain mitochondrial function and oxidative phosphorylation activity during BMSC maturation into bone-producing cells. Suppression of NAD+ by blocking NAMPT impaired mitochondrial fusion, leading to mitochondria dysfunction and reduced oxidative phosphorylation activity, which subsequently stopped bone production.
Since NMN is the immediate product of NAMPT, Li and colleagues investigated whether NMN could alleviate the inhibition of NAMPT on osteoblast generation and rescue mitochondrial function. NMN treatment partially recovered the osteoblast generation and bone formation suppressed by inhibiting NAMPT. Of note, NMN markedly prevented the reductions in mitochondrial health and function caused by NAMPT inhibition. d. These data indicate that blocking NAD+ synthesis by inhibiting NAMPT suppresses BMSC osteogenesis, which could be partially recovered by replenishing NMN.
Bone Repair in Mice Depends on NAD+ Production via NMN
To evaluate the role of NAD+ in osteogenesis in mice, Li and colleagues conducted a bone fracture model experiment and injection with the NAMPT inhibitor FK866. Consistent with the cell culture data, the Sichuan University researchers show that fracture healing is repressed with the impaired formation of cartilage and bone when NAMPT is inhibited. Also, the mineral density of the callus – the bony and cartilaginous material forming a connecting bridge across a bone fracture during repair – was reduced with the FK866 treatment.
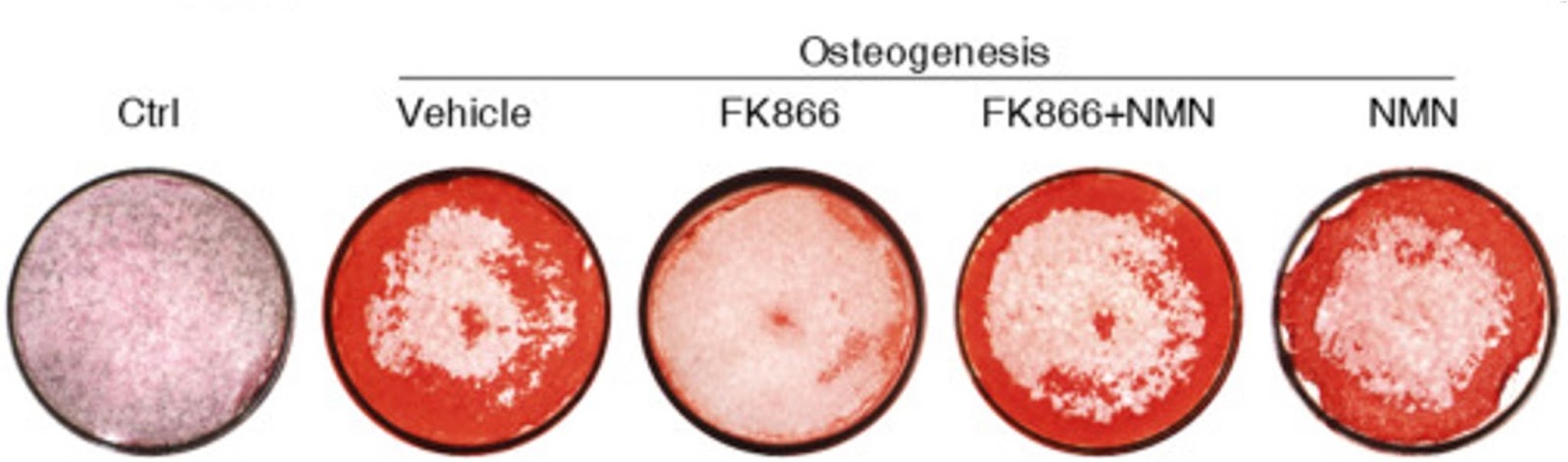
(Li et al., 2022 | Stem Cell Research & Therapy) NMN is required for adult stem cells to generate bone. Osteogenesis – the formation of bone-producing cells (stained red) – from human BMSCs is blocked by the NAMPT inhibitor FK866. NMN, which is the output of NAMPT activity, overcomes FK866 inhibition and recovers the osteogenesis capacity of BMSCs.
Can NAD+ Boosting Improve Bone Repair?
It will be interesting to examine whether replenishment of NAD+ or its intermediates benefits bone fracture repair. The work of Li and colleagues is consistent with other recent work on NMN and bone health and production. For example, a study published just weeks earlier showed that NMN can reinvigorate the “stemness” of BMSCs by preventing senescence – an age-related condition where cells no longer grow or replicate. And in 2020, another study revealed a potential connection between NMN treatment as a remedy for osteoporosis in aging mice. This confluence of data may push someone to take the plunge into looking at the therapeutic potential of NMN in bone formation and regeneration in humans.

