New Stem Cell–Derived Immune Cells Reverse Memory Loss in Aging and Alzheimer’s Brains
Stem cell–derived immune cells improved memory and brain health in aging and Alzheimer’s mice by reducing inflammation and supporting neural repair.
Highlights
- Scientists engineered replacement immune cells from human stem cells and implanted them into aging and Alzheimer’s disease mouse models.
- The new cells improved memory, reduced inflammation, and restored healthier neural structure in key brain regions.
- Treated animals showed better performance on cognitive tests, suggesting that immune-cell rejuvenation may support healthy brain aging and counteract neurodegeneration.
Aging is strongly linked to chronic brain inflammation, reduced clearance of debris, and declining neural repair. These changes contribute to cognitive loss and raise the risk of Alzheimer’s disease. Many older adults spend years living with impaired memory and reduced resilience. Extending healthspan, meaning the years lived in good health, will likely require strategies that protect the brain’s immune and repair systems, which begin to fail long before major symptoms appear.
In a new Advanced Science study, researchers tested a regenerative approach. They generated fresh mononuclear phagocytes – immune cells such as monocytes and macrophages that act as the body’s cleanup and repair crew – from human induced pluripotent stem cells. Induced pluripotent stem cells, often abbreviated as iPSCs, are adult cells that scientists reprogram back into a flexible, stem-like state. The team then directed these stem cells to become phagocytes, a family of immune cells that clear waste, regulate inflammation, and support tissue repair.
These stem cell–derived immune cells were introduced into the bloodstream of aging mice and mice modeling Alzheimer’s disease. After infusion, the cells migrated into the brain and began to perform roles similar to microglia, the brain’s resident immune cells.
Replacing Faulty Immune Cells Improved Memory and Learning
The treated mice performed significantly better on learning and memory tasks than untreated controls. Aging normally weakens microglial function, which allows inflammatory molecules and cellular debris to build up around neurons. That buildup interferes with the formation and maintenance of synapses, the contact points where neurons communicate.
The implanted phagocytes restored more youthful immune activity in the brain. They improved debris clearance, reduced inflammatory signaling, and helped preserve synaptic structure. This result strengthens a growing idea in aging research. When the brain’s immune cells lose efficiency, cognitive performance tends to decline because waste accumulates, inflammation rises, and neurons lose support. The transplanted cells reversed part of that pattern by taking over essential cleanup and repair roles, which helped stabilize neural circuits that usually weaken with age.
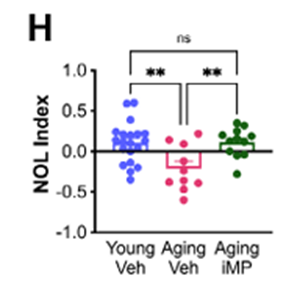
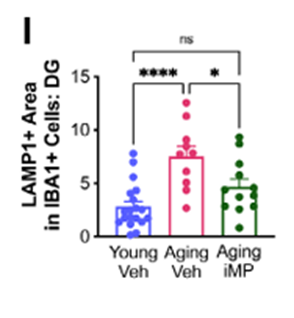
New Immune Cells Reduced Inflammation and Supported Neural Repair
Across multiple mouse models, the transplanted phagocytes consistently lowered inflammatory activity in the brain. Chronic inflammation disrupts communication between neurons and accelerates neurodegenerative processes. After treatment, the animals showed reduced levels of inflammatory markers and stronger activation of molecular pathways involved in cellular repair and maintenance.
Microscopy revealed healthier synaptic density and better structural integrity in key brain regions. Neurons maintained more of their branch-like extensions, and the connections between cells appeared more robust. These structural changes matched the behavioral improvements seen in the cognitive tests and suggest that the treatment shifted the brain toward a more resilient state, rather than providing a brief or superficial benefit.
Alzheimer’s Models Showed Reduced Disease Burden
The researchers also tested the approach in mouse models that mimic important features of Alzheimer’s disease. In these animals, the stem cell–derived phagocytes moderated several disease-related changes. The new cells removed amyloid-beta deposits (a hallmark of Alzheimer’s) more efficiently, improved local metabolic conditions, and reduced stress signals in affected brain regions.
The intervention did not cure Alzheimer’s, and the disease processes did not disappear entirely. However, the treated animals showed less accumulation of damaging proteins and better preservation of neural function. These results suggest that immune-cell replacement can influence several pathways that contribute to Alzheimer’s pathology at the same time.
Why These Findings Matter for Brain Aging
The study adds weight to an emerging view that healthy brain aging depends heavily on immune function. Microglia do much more than respond to infection. They shape synapses, control inflammatory tone, and clear toxic proteins throughout life. When these cells become dysregulated with age, the brain loses a major line of defense.
By introducing healthier, stem-cell–derived phagocytes, the researchers partially restored these protective functions in aging and Alzheimer’s mice. The work suggests that supporting or replacing the brain’s immune cells may offer a way to maintain cognition and slow neurodegenerative processes without directly altering neurons themselves.
At the same time, the authors emphasize that the findings are still early-stage. All of the evidence comes from mouse models. Human brains have more complex immune environments, and long-term safety remains unknown. Future studies will need to test how long the transplanted cells remain beneficial, whether they integrate safely over time, and whether similar effects appear in larger animal models or human-derived brain systems.
Even with these caveats, the study marks a meaningful step in longevity and neurodegeneration research. Rejuvenating the brain’s immune network may eventually become part of a broader toolkit for protecting cognitive health. If that vision holds up in further work, interventions that restore immune support in the brain could help shift more years of life from decline toward preserved function and independence.

