New Intranasal and Injectable Gene Therapy Extends Lifespan in Mice
A preprint article shows safe and effective viral gene therapy strategies that promote healthier aging and extend longevity
Highlights
· This is the first report of researchers using cytomegalovirus successfully as both an intranasal and injectable gene therapy system to extend longevity.
· The treatment significantly improved glucose tolerance, physical performance, and prevented loss of body mass and alopecia.
· Intranasal and injectable preparations performed equally well in delivering gene therapy to multiple organs, with long-lasting benefits, and without unwanted side effects.
Whether it’s in a naturally occurring chemical or a technology created in a lab, the search is on for safe and effective means of mitigating the impact of aging on human health.
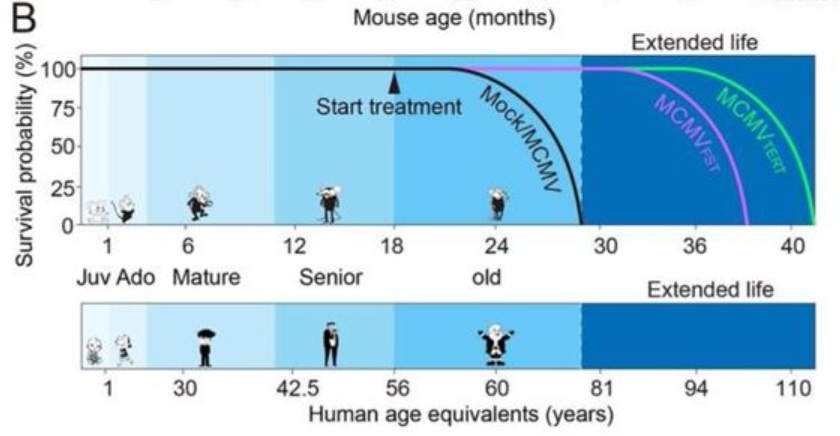
New research led by investigators at Rutgers New Jersey Medical School shows that gene therapy delivered via injection or intranasally extends lifespan, improves physical performance, and reduces the age of cells in mice. These results, which are yet to undergo peer review, show that viruses that deliver and drive the activity of two genes, telomerase reverse transcriptase (TERT) and follistatin (FST), extended median mouse lifespan by 41.4% and 32.5%, respectively.
“Translating this research to humans could have significant benefits associated with increased healthspan,” proposed Jaijyan and colleagues.
Candidates for gene therapy aimed to improve healthy lifespans
How to achieve healthy longevity has remained a challenging subject in biomedical science. One school of thought is based on reversing molecular or cellular events that drive aging. For example, aging is linked to shortening telomeres — the protective DNA caps of chromosomes. This happens, in part, because of insufficient activity of an enzyme called telomerase reverse transcriptase (TERT) that maintains telomere length. Animals deficient in TERT have shorter telomeres, shorter lifespans, and an increased risk of age-related diseases like heart disease. Recent studies on animal models have shown the therapeutic efficacy of TERT in increasing healthy longevity and reversing the aging process.
Another avenue pursued by investigators trying to stave off aging has been to promote processes that are typically active during development and youth. For example, the follistatin (FST) gene plays a key role in muscle development. Increasing FST levels is known to increase skeletal muscle mass in transgenic mice by two- to three-fold. Mice lacking FST have smaller and fewer muscle fibers and show stunted growth, skeletal defects, reduced body mass, and die in a few hours after birth, suggesting an important role of FST in skeletal muscle development. These findings strongly implicate the therapeutic potential of FST in the treatment of muscular dystrophy and muscle loss caused by aging or microgravity.
What’s the best gene therapy strategy?
As more longevity-supporting factors are discovered, it is of interest to determine potential large capacity viruses, also called vectors, for delivering multiple genes simultaneously. Cytomegaloviruses (CMV) — part of the herpesvirus family but which rarely cause health problems in humans — are attractive vehicles for gene therapy because they do not integrate their DNA into the host genome, mitigating the risk of causing mutations, genome instability, and malignancies.
These viruses are classified by the host species they can infect. For example, there are CMVs that infect mice (MCMV) as well as CMVs that infect humans (HCMV), which have proven to be a safe method for gene therapy in human clinical trials.
Cytomegalovirus-based gene therapy extends mouse lifespan
Using MCMV as a viral vector, Jaijyan examined the therapeutic potential of TERT and FST gene therapy to offset biological aging in a mouse model. These vectors were delivered intraperitoneally (IP) or intranasally (IN) and evaluated by how much TERT or FST protein these gene therapy vectors could produce daily.
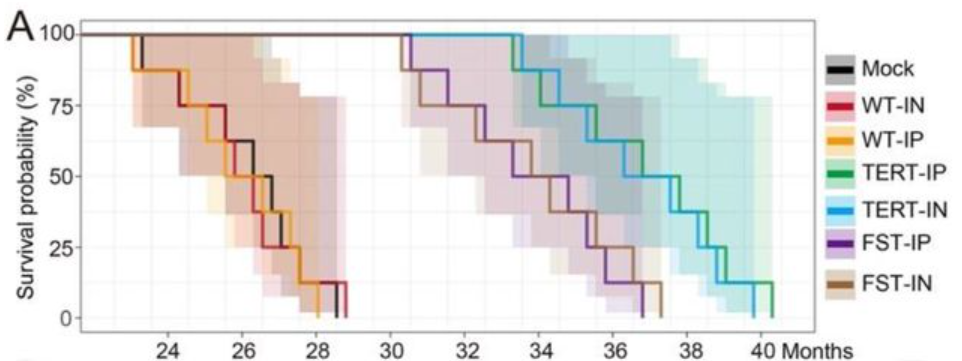
Consistent with previous reports on the lifespan of mice, the Rutgers researchers found that the mice that either didn’t receive any virus (mock) or received an “empty” MCMV had a median lifespan of about 26 months. For the groups treated with MCMVs carrying the FST gene, the median age at death was roughly 35 months (over 30% increase), while mice treated with MCMVs carrying the TERT gene lived around 37 months (over 40% increase).
Although this was not the first time that viruses have been used to extend the lifespan of mice, it was the most effective. Previous studies showed that adenovirus-associated viruses (AAV) — small viruses that infect humans and some other primate species — carrying the TERT gene can suppress or reverse the effects of aging in mice. But the results with MCMV far exceed the longevity achieved with TERT gene therapy using AAV, which was 13% and 24% when delivered in a single dose at 2- and 1-year-old mice, respectively.
MCMVs driving TERT and FST improved activity and motor coordination
Not only did this gene therapy strategy promote longevity, but it improved the physical and physiological conditions of these mice. Using a beam-crossing coordination test to assess motor control, mice treated with MCMVTERT or MCMVFST completed the balancing act in ~7.5 and 12.5 seconds, respectively. These results were vastly improved compared to that of controls, which took about 43 seconds, demonstrating that the gene therapy strategy in question yielded mice with superior coordination.
FST treated mice showed an increase in body mass, which was not too surprising given that previous studies that increased FST activity by different means provided similar results. Jianjyan and colleagues observed that skeletal muscle mass was indeed larger than that of controls. The increased robustness may explain the improved motor control in executing the beam test.
“We anticipate that sarcopenia, muscular dystrophy, or even special circumstances causing muscle atrophy, such as low gravity exposure during space travel, could be mitigated with a CMV-based FST gene delivery method,” proposed Jianjyan and colleagues.
MCMVs driving TERT and FST increased glucose tolerance
Glucose tolerance is known to decrease with aging. Using a glucose tolerance test in fasted mice from each treatment group, Jianjyan observed that the average peak glucose concentration was ~33% lower for TERT and ~28% lower for FST treatments than for controls.
Moreover, blood sugar levels reached baseline one hour post-administration in mice treated with MCMVs delivering TERT and FST, in contrast to ~7 hours for control mice. These results show that TERT and FST treatments were equally effective in blood glucose processing.
MCMV gene therapy prevents mitochondrial deterioration in mice
The relative telomere length in the heart, liver, kidney, brain, lung, and muscle in 24-month-old mice treated with the MCMV carrying the TERT gene was 6-fold greater than in control mice of the same age. Indicators of healthy mitochondria — cell structures that provide essential metabolic support — showed improvements in both aged heart and skeletal muscle. Specifically, the gene therapy strategies in question increased the number of mitochondria with connected cristae — indicative of functionality — and the mitochondrial area compared to control aged mice and reached levels seen in young mice.
“Using mouse cytomegalovirus (MCMV) as a viral vector, we examined the therapeutic potential of TERT and FST gene therapy to offset biological aging in a mouse model, and demonstrated significant lifespan increase, as well as positive metabolic and physical performance effects,” concluded Jaijyan and colleagues. “Our study justifies further efforts to investigate the use of CMV TERT and FST vectors against aging-related chronic inflammatory conditions, type 2 diabetes, sarcopenia, dementia, lung, kidney, and heart diseases responsible for decreased quality of life and premature death.”
Are these gene therapy strategies the antidotes to human aging?
Though we often look for therapeutic strategies that require just one treatment, the therapeutic regimen here required monthly administration to have continuous effects. But the fact that this gene therapy strategy requires repeated administration may be advantageous to achieve a reduced risk of long-term consequences in case of adverse reactions, should any occur. Nevertheless, we need studies to determine whether an uninterrupted monthly administration has a different outcome in longevity extension.
Everything said aside, since this study has not yet been peer reviewed, everything stated in this article should be taken with a grain of salt. Until these results are accepted in a peer-reviewed journal, let alone shown to translate into humans, these results absolutely should not guide health-related behavior.

