Life-Extending Gene Therapies: Successes, Failures, and Secrets
A new study lays the groundwork for life-extending gene therapies for humans, while past studies have done so less.
Highlights:
- A promising new, possibly life-extending, gene therapy has been shown to reduce the symptoms of heart disease and improve exercise performance.
- Past age-reversing gene therapies were studied in the shadows of government regulation and have been called into question.
The latest results from the EXACT Phase II trial show that a new gene therapy reduces the symptoms of coronary artery disease (CAD) — the most common form of heart disease in the United States. Furthermore, the gene therapy improves the exercise capacity of CAD patients while counteracting impairments in blood flow within the heart.
The data was presented at the Society for Cardiovascular Angiography & Interventions (SCAI) 2024 Scientific Sessions in Long Beach, California. The gene therapy, named XC001, delivers VEGF (vascular endothelial growth factor), which promotes the formation of new blood vessels. Given that VEGF gene therapy was previously shown to prolong the lifespan of mice, XC001 may prove to be the first successful life-extending gene therapy for humans.
Bypassing Clogged Arteries by Generating New Routes
Our coronary arteries wrap the surface of our heart, providing our heart tissue with oxygenated blood. However, when plaque builds up (atherosclerosis) in our coronary arteries, it causes CAD. Large enough plaques can limit blood flow, causing severe to debilitating chest pain called angina. Moreover, these plaques can rupture, block the artery, and trigger a heart attack.
Hypothetically, blood flow to the heart could be increased in CAD patients by triggering the growth of new blood vessels and bypassing the narrowed arteries. This could be done by artificially increasing VEGF, long known to stimulate the formation of new blood vessels. By increasing VEGF levels at the surface of the heart, the signaling factor could induce the growth of tiny new blood vessels called capillaries that branch off from the coronary arteries.
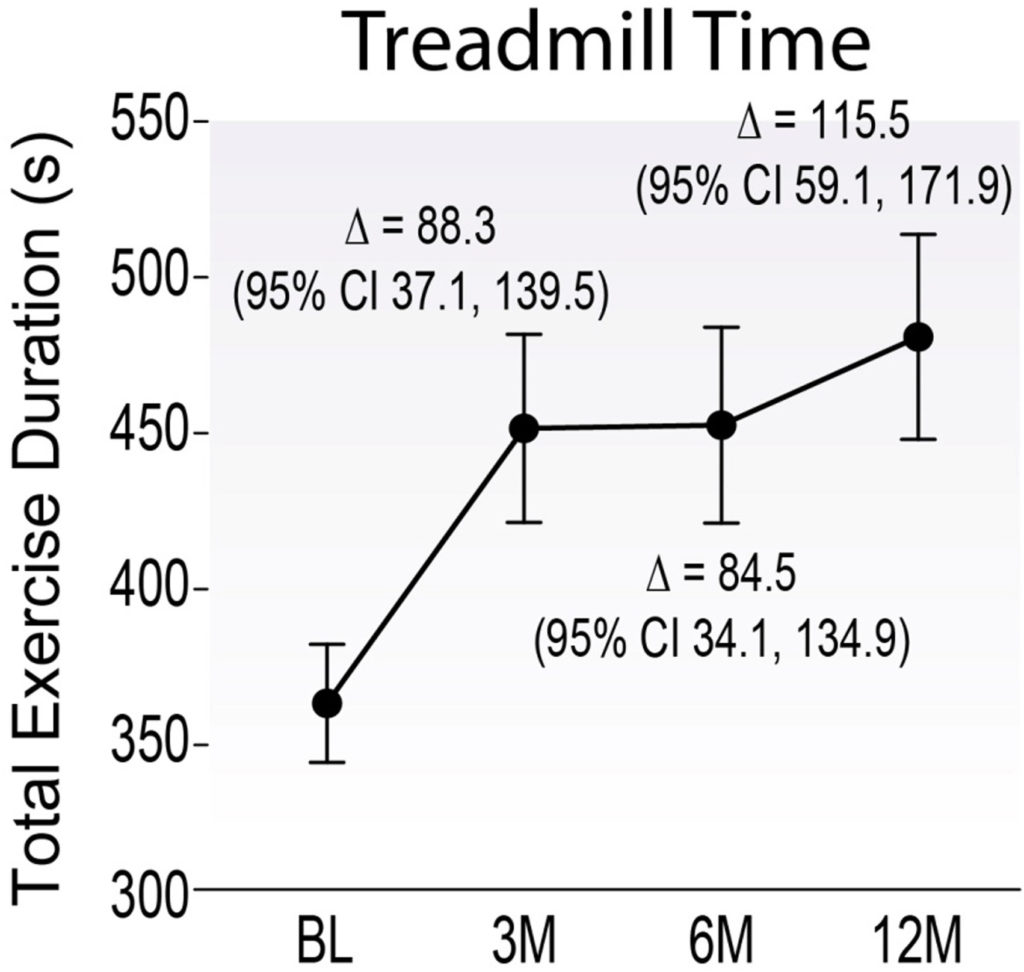
This seems to be exactly what the EXACT trial team did. XC001 utilizes a non-pathogenic adeno-associated virus (AAV) to deliver VEGF to cells. Relatively low concentrations of XC001 (VEGF-containing AAV particles) were injected into the surface of the heart. This led to an increase in blood flow to the hearts of CAD patients, who then experienced less angina and were able to run on a treadmill for a longer duration. These outcomes suggest the formation of new blood vessels (angiogenesis), allowing the heart to receive more oxygenated blood.
Age Reversal Gene Therapies Called into Question
“They seem really cryptic,” Leigh Turner, a bioethicist at the University of California, Irvine, told WIRED. “The details that have come out have not been reassuring in terms of the credentials and qualifications of clinicians involved, the clinical facilities that people go to, or the protocols that are in place.”
Dr. Turner was speaking of BioViva, a secretive company working outside of the FDA’s reach in places like the British Virgin Islands and Tijuana, Mexico. BioViva is credited with being the first company to use a gene therapy to reverse aging. This gene therapy was tested on a single person — the CEO and founder of BioViva, Liz Parrish. The results of this case study showed that a telomerase — an enzyme that prolongs telomeres — gene therapy reversed Parissh’s biological age.
Scientists, particularly Charles Brenner, Ph.D., have called these results into question, saying that biological age is not an accepted measurement of age reversal. He also has doubts about another study published by BioViva, which showed that a gene therapy combination of telomerase and klotho — a pro-longevity gene — reversed dementia symptoms.
“It’s not established that telomerase activity limits human healthspan, and cognitive impairment is not very well understood,” said Brenner. “The experiment that was done is not capable of producing meaningful results. I wouldn’t expect it to work, and I doubt that it does.”
Additionally, BioViva’s studies were published in the Journal of Regenerative Biology and Medicine, which had a meager impact factor of 0.571 for 2021-2022. An impact factor reflects how often the articles within a journal are cited and is a metric of credibility. For comparison Nature’s 2022 impact factor was 64.8 while Science’s impact factor was 56.9. Also, the editor-in-chief of the Journal of Regenerative Biology and Medicine sells penis enhancement injections in Cancun, Mexico called I-Guana Shots.
The First Life-Extending Gene Therapy?
BioViva aside, XC001 appears to be the most promising gene therapy targeting aging to date. Still, it does not come without limitations. For example, XC001 was injected directly into the heart, requiring expensive, dangerous, and invasive surgery. The need to specifically target heart tissue brings up another problem with gene therapies. That is, we have not yet perfected technologies to target specific tissues and cells without local injections.
Otherwise, we could inject gene therapies intravenously (IV), which is far less invasive than open heart surgery. And targeting specific tissues is important because off-target effects can be catastrophic. For example, if VEGF were to be elevated in the retina, it could trigger a disease called wet macular degeneration, where new blood vessel formation can lead to blindness. Still, invasive surgeries may be worthwhile, especially in the case of CAD, which is the leading cause of death in the United States.
It’s quite possible that the 32 patients treated with XC001 will live longer than they otherwise would have. However, there was no control group to compare to the patients who received the gene therapy. A Phase III clinical, which may include a control group, could measure heart attack and mortality incidence following XC001 treatment to help confirm whether the gene therapy is likely to lower the risk of cardiovascular-related mortality. If so, XC001 could be the first life-extending gene therapy.

