Mayo Clinic Study Indicates Dasatinib Provides Antidiabetes Benefits in Humans
Dasatinib may improve diabetes symptoms compared to contemporary diabetic treatments and may be considered for novel diabetic therapy usage.
Highlights
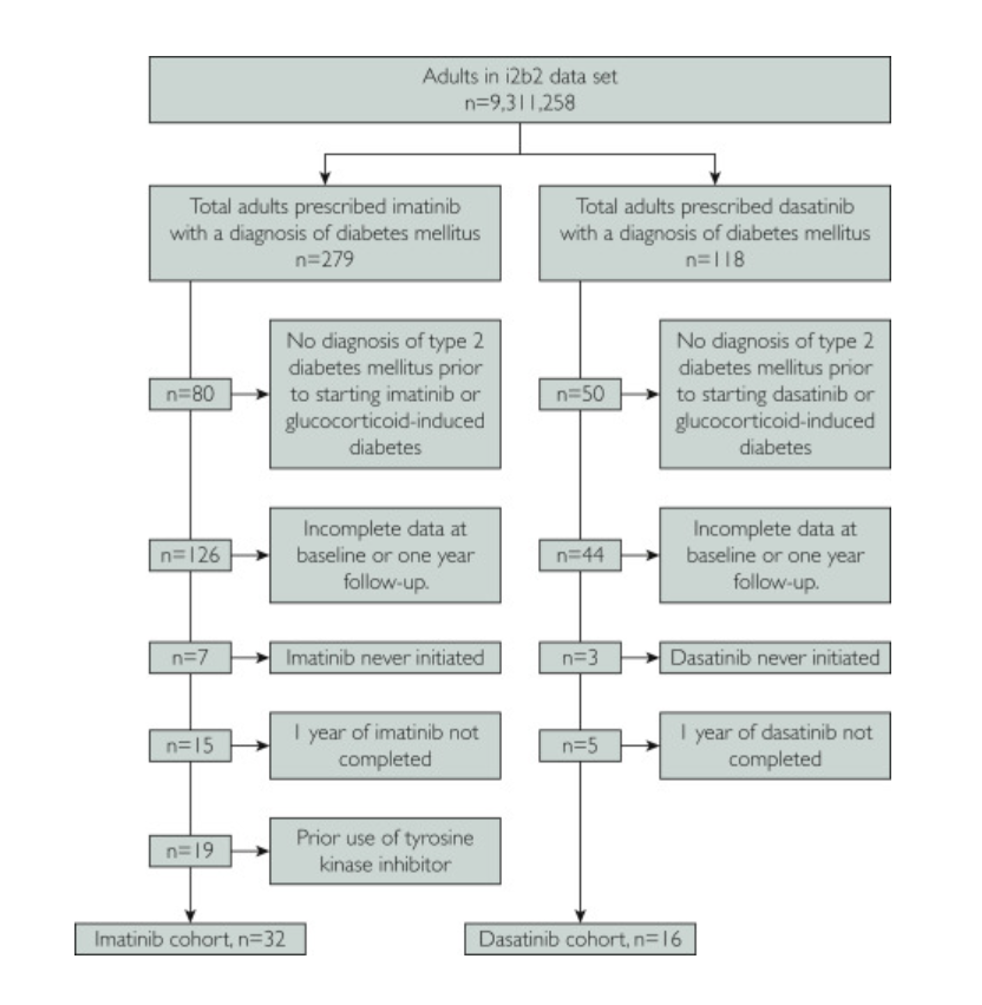
- The study screened over 9 million patients with type II diabetes and an underlying predisposition to cancer.
- Dasatinib lowered measurements of type II diabetes severity, like sugars in the blood, in the research conducted over the course of 26 years.
- This study provides clues that, by targeting aged, non-proliferating cells (senescent cells), we may be closer to effectively combating diabetes.
A plethora of compounds that eliminate aged, non-proliferating cells termed senescent cells are currently at our disposal – at least in animals like mice. But finding out whether they work in humans has remained, for the most part, unexplored. One senolytic agent with anticancer properties, dasatinib, has been shown to prevent obesity in mice, likely by eliminating senescent cells. Since obesity drives type 2 diabetes, researchers have sought to find whether the senolytic dasatinib can improve signs of type 2 diabetes, like elevated blood sugars, in humans.
Pignolo and colleagues from the Mayo Clinic published in Mayo Clinic Proceedings showing that dasatinib taken orally may provide antidiabetic effects in people with type 2 diabetes, comparable to current treatments for diabetes. The Rochester-based team analyzed dasatinib’s effects in type II diabetes patients with a predisposition to cancer and found that the senolytic significantly diminishes the sugar glucose in the blood. The research team compared dasatinib’s antidiabetic effects to a weaker senolytic called imatinib to show that dasatinib’s influence on the age-related disease comes from its potential senolytic properties.
“This study was really the first proof-of-concept that a senolytic drug may have substantial long-term beneficial effects in humans,” said Dr. Pignolo in a press release on the study.
Diabetes Patients Were Analyzed for 26 Years While Taking Dasatinib
To analyze dasatinib’s possible antidiabetes properties, the Mayo Clinic researchers used diabetes patients examined for approximately 26 years — from January 1994 through December 2019. To measure how strong dasatinib’s possible effects are, they split the diabetes patients into two groups – one treated with dasatinib and another treated with a weak senolytic called imatinib. Pignolo and colleagues then looked at hemoglobin A1C, a measurement of sugars in the blood and levels of glucose in the blood as shown by serum glucose concentrations.
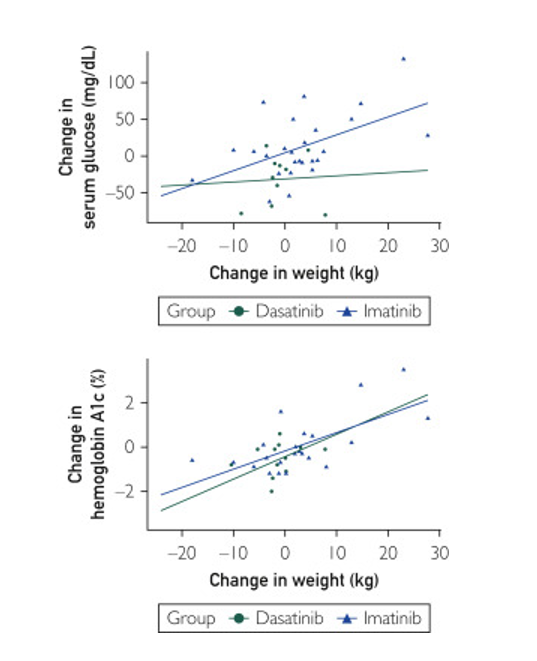
Dasatinib Lowers Blood Sugars Like Glucose in Diabetes Patients
The research team got some intriguing results. For example, compared to the diabetic patient group that received imatinib, those receiving dasatinib had a significant drop in serum glucose concentration of 43.7 mg/dL (P=.005). The lowered serum glucose levels in the dasatinib group facilitated a trend toward less insulin required for their diabetes (P=.08 with 28.8 fewer total daily insulin units). The lowered serum glucose in the dasatinib group occurred in the context of a 4.8 kg (~10.6 pounds) weight loss. A statistical analysis suggested that the weight loss accounted for 19.2% of the 43.7 mg/dL dip in serum glucose concentrations in the diabetes patients treated with dasatinib.
Hemoglobin A1C blood sugar measurements indicated that, relative to imatinib, dasatinib-treated patients had a significant reduction in blood sugars (P=.05). This finding correlated to the diabetes patients requiring less insulin — 18.2 daily units of insulin less per day (P=.16) in the setting of 5.9 kg (~13 pounds) of weight loss.
“Our findings suggest that dasatinib or related senolytic drugs may become novel diabetic therapeutics,” said Pignolo and colleagues. “More study is needed to determine whether these findings also are observed in patients with type 2 diabetes mellitus but without underlying malignant disease.”

Dasatinib and/or the Supplement Fisetin May Work Against Diabetes
Future studies need to determine whether these benefits translate to diabetes patients without a predisposition to cancer (underlying malignant disease). Research also needs to unravel whether dasatinib’s antidiabetic effects are due to its ability to prune and eliminate aged, non-proliferating senescent cells. If dasatinib does, in fact, provide these benefits, combining it with other senolytics like quercetin may enhance dasatinib’s capabilities in combating diabetes.
Fisetin may be another senolytic that patients can use in combination with dasatinib. This natural flavonoid has been shown to have similar benefits to dasatinib and quercetin in mice with an aging disease (progeroid mice).
“If the glycemic benefit of dasatinib is indeed through senescence clearance, fisetin is an attractive alternative senolytic in future clinical trials,” said Pignolo and colleagues. So, another perspective is that the supplement fisetin on its own could provide a more cost-effective means of combating diabetes. A problem with this view is that no clinical trials showing that fisetin works in diabetes patients have been completed.
Whatever the case may be, the future looks good for applying new therapeutics like dasatinib or fisetin for use against diabetes. The results show that dasatinib likely works against diabetes, at least in diabetes patients with underlying malignant disease. As the old adage goes, “I think we’re onto something.”