Mayo Clinic Study Shows Spinal Cord Nerves Depend on NAD+
In mice, high-fat diets cause the degradation of conductive nerve coatings called myelin, but they can be regenerated by restoring NAD+ levels with the precursor NMN.
Highlights
· Diets high in fat adversely impact the survival and function of myelin-producing cells called oligodendrocytes by reducing NAD+ levels in mice.
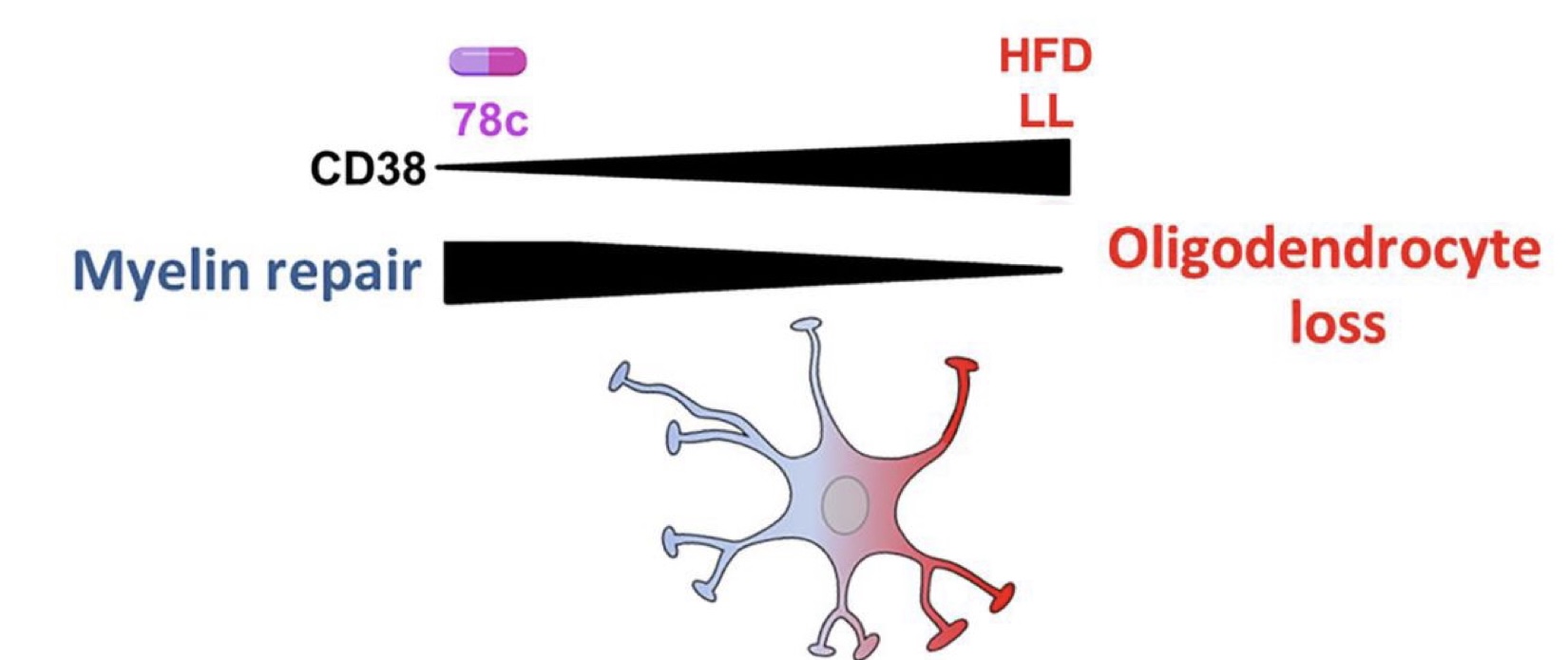
· Inactivating CD38 — an NAD+ consuming enzyme — by genetic or drug-based manipulation enhances oligodendrocyte survival.
· Restoring NAD+ levels with the NAD+ precursor NMN prevents oligodendrocyte loss and promotes myelin repair.
For conscious and subconscious tasks, from giving a hug to breathing, our nervous system needs to send electric signals at lightning speed. To achieve biological fiber-optic-like transmission, our nerve cells called neurons are insulated in myelin — a conductive cover for speedy nerve communication. But when myelin is disrupted, which happens with age, certain genetic conditions like multiple sclerosis, and poor metabolism, we become susceptible to permanent spinal cord and brain damage. So, all that fast food and inactivity isn’t just bad for your metabolism; it can deteriorate myelin coating to severely weaken nerve signals essential for everyday life.
Research from the Mayo Clinic published in The Journal of Neuroscience shows that the white matter, where myelin-wrapped nerve projections run through the brain and spinal cord, can be impaired by high-fat consumption. This occurs by disrupting levels of a vital metabolic coenzyme called nicotinamide adenine dinucleotide (NAD+), leading to the loss of nervous system cells called oligodendrocytes — cells that add myelin to neurons. But increasing NAD+ levels, either by blocking the consumption of NAD+ or supplementing with the precursor nicotinamide mononucleotide (NMN), attenuated oligodendrocyte loss and promoted myelin regeneration.
“These findings point to a new metabolic targeting strategy positioned to improve disease course in multiple sclerosis and other conditions in which myelin integrity is a key concern,” said the authors.
Relevance of the Diet and NAD+ to Multiple Sclerosis
Western-style diets cause disruptions in myelinating cells called oligodendrocytes and supporting cells called astrocytes. Obesity is a crucial risk factor for the development, progression, and disability burden of multiple sclerosis — a disease in which the immune system eats away at myelin, disrupting communication between the brain and the body.
CD38, the main NAD+ depleting enzyme in the nervous system, has increased levels in models of multiple sclerosis. Altered NAD+ metabolism is linked to both high-fat consumption and multiple sclerosis. Also, several recent studies explored supplementing NAD+ precursors or plant-derived compounds known as flavonoids known to inhibit NAD+-depleting enzymes from impeding myelin repair and neuroprotection. For example, NAD+ precursor supplementation has had therapeutic remyelinating effects in various models of neurological diseases, such as stroke and Huntington’s disease.
CD38 silenced mice resist oligodendrocyte loss caused by a high-fat diet
In this study, Langley and colleagues from the Mayo Clinic linked diet, myelin, and NAD+ together. They found increased CD38 levels and decreased NAD+ levels in mouse spinal cords following chronic high fat consumption, demyelinating injury, and active multiple sclerosis-like lesions — an area of damage or scarring (sclerosis) in the spinal cord and brain. But mice with genetically or therapeutically inactivated CD38 were substantially protected from high fat-induced NAD+ depletion and oligodendrocyte loss.
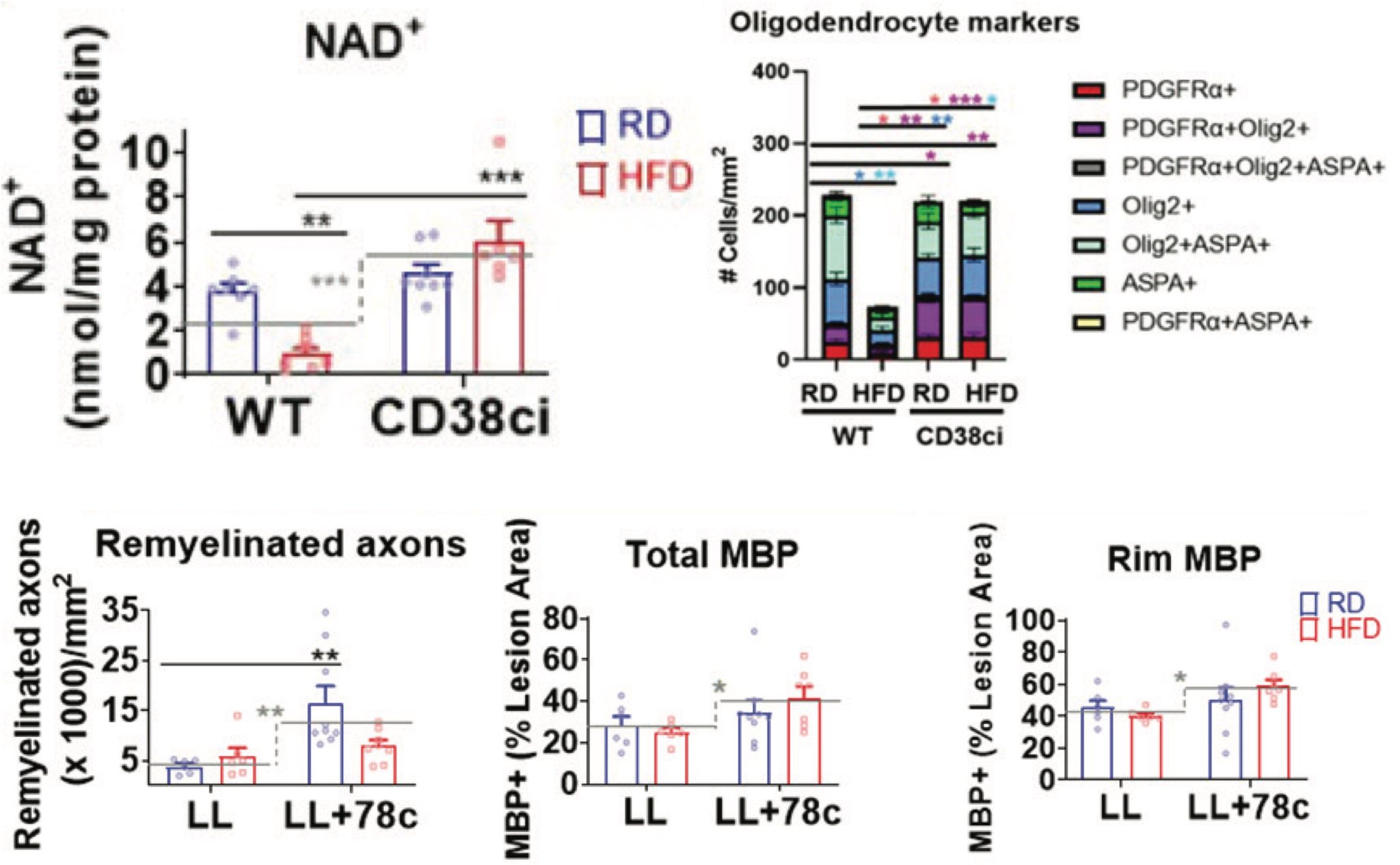
NAD+ and NMN restore oligodendrocyte differentiation in fat-saturated cultures
To examine the effects of replenishing NAD+ levels on the nervous system, Langley and colleagues next supplemented oligodendrocyte cultures with NAD+ or NMN. While saturated fat alone reduces the levels of critical oligodendrocyte and myelin proteins, co-treatment with NAD+ or NMN recovered these levels to that of untreated cells. These findings suggest that increases in astrocyte CD38, and the associated depletion of NAD+ levels, may contribute indirectly to impairments in oligodendrocyte health and myelin regeneration via inflammation and oxidative stress.
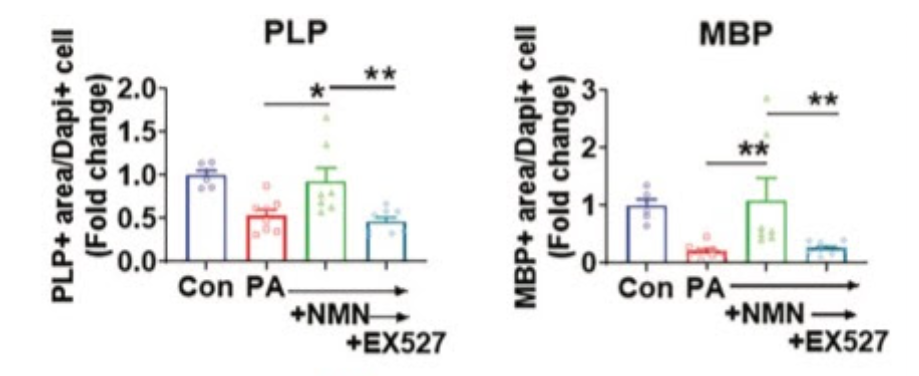
“Our findings suggest high-fat diet impairs oligodendrocyte survival and differentiation … mediated by the NAD+-consuming enzyme CD38 and highlight CD38 inhibitors as potential therapeutic candidates to improve myelin regeneration,” said Langley and colleagues.
They also say that these studies are the basis for future research to elucidate the role of diet on oligodendrocytes during development and aging.
Together these findings point to the potential translational value of targeting CD38 to improve outcomes in multiple sclerosis and other demyelinating conditions.
The authors propose, “The research findings we present should inspire further research into ways to mitigate the negative consequences of high-fat diets on oligodendrocytes alone and in the context of white matter injury which may include dietary intake of NAD+ precursors, administration of CD38 inhibitors, or exercise-related rehabilitation strategies.”