MIT Researchers Show Key Brain Cells Age Prematurely in Down Syndrome
Anti-aging drugs may be an exciting new therapeutic avenue for treating Down syndrome patients
Highlights
- The third copy of chromosome 21 disrupts the nuclear architecture and genome organization in neural progenitors — a key cell type of the developing brain.
- Neural progenitor cells harboring three copies of chromosome 21 display signatures of an aging-associated state known as cellular senescence.
- Drugs targeting senescent cells (senolytics) ameliorate trisomy-21-associated dysfunctions in neural progenitor cell populations.
Down syndrome is the most common chromosomal disorder, typically characterized by moderate to severe intellectual disability associated with abnormal brain development. While life expectancy of Down syndrome population has greatly increased over the last decades, mortality rates are still high and subjects are prematurely facing atypical and accelerated aging.
Research from MIT indicates that senescence — a phenomenon describing the loss of a cell’s power of division and growth commonly linked to aging — may play a key role in Down syndrome by disrupting brain development. The third copy of chromosome 21 disrupts the 3D organization of the genome in key brain cells during development, leading to altered gene activation patterns that drive senescence. Published in Cell Stem Cell, Meharena and colleagues show that drugs that eliminate senescent cells called senolytics provide an exciting therapeutic avenue for treating individuals with Down syndrome by restoring brain cell dysfunctions.
“That senescence might be playing a role in the neuropathology of Down syndrome is very interesting,” said Tamir Chandra, a molecular geneticist at the University of Edinburgh who was not involved in the new study. “That opens a new door for exploring potential interventions.”
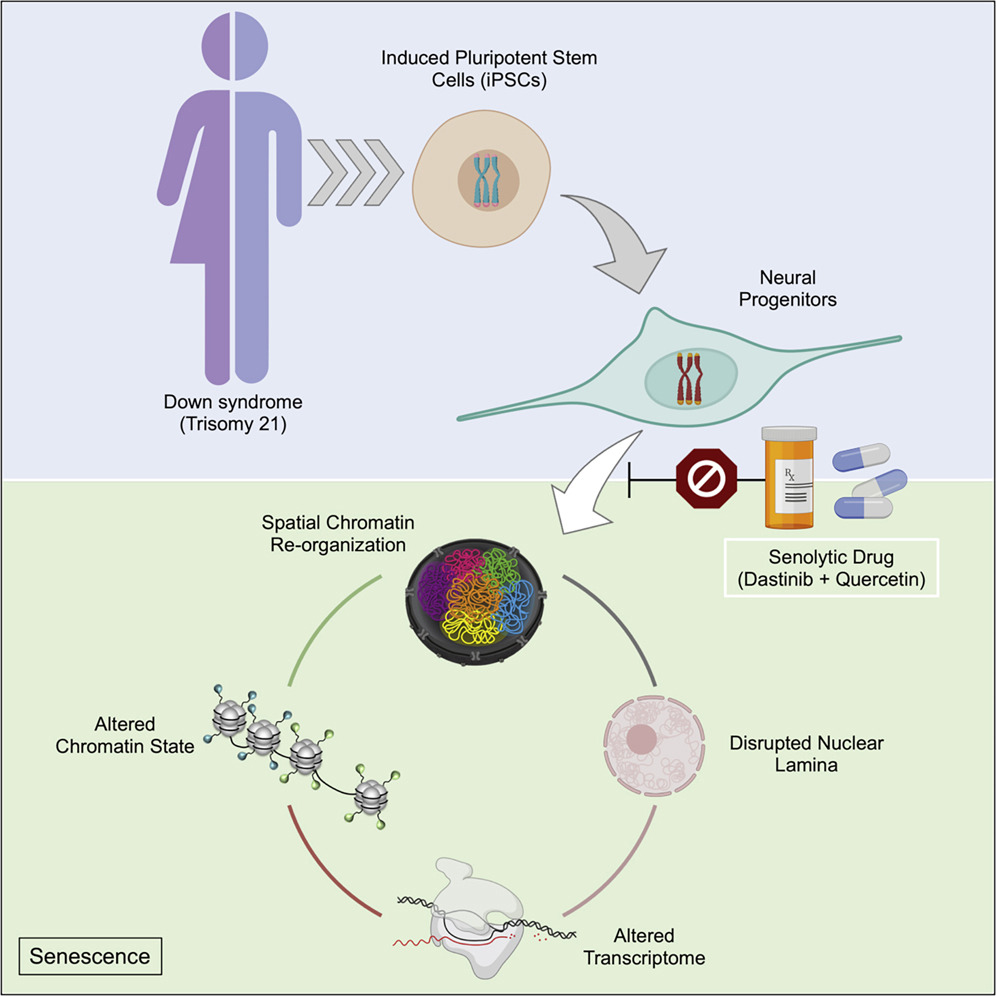
(Meharena et al., 2022 | Cell Stem Cell)Unlike human induced pluripotent stem cells (iPSCs), iPSC-derived neural progenitor cells (NPCs) exhibit gene activation and nuclear architecture changes characteristic of senescent cells in response to trisomy 21. Treatment of trisomy-21-harboring NPCs with senolytic drugs alleviates the transcriptional, molecular, and cellular dysfunctions associated with Down syndrome. These findings provide a mechanistic link between T21 and global transcriptional disruption and indicate that senescence-associated phenotypes may play a key role in the neurodevelopmental pathogenesis of Down syndrome.
How does an extra chromosome drive Down syndrome?
Down syndrome is a genetic disorder driven by the triplication of chromosome 21 (T21) and characterized by a wide range of neurodevelopmental and physical disabilities. These brain abnormalities have been associated with dysfunctional neural progenitor cells (NPCs) — the multipotent stem cells of the developing brain that differentiate into information sending nerve cells (neurons) and their supporting cells (astrocytes and oligodendrocytes). But how this occurs as a consequence of T21 has been unknown.
Studies utilizing human-derived tissue samples and Down syndrome mouse models have established that T21 induces global dysregulation of gene activation. The three-dimensional organization of the genome within the nucleus determines the levels of specific gene activity within a cell. However, the influence of T21 on the 3D-genome organization and the global transcriptional disruption observed in Down syndrome had been unexplored.
T21 induces chromosomal introversion in key brain cells
To decode the molecular mechanisms underlying these global gene changes, Meharena and colleagues utilized cells from Down syndrome patients that were reprogrammed into stem cells and then turned into NPCs. The MIT researchers observed chromosomal introversion in NPCs harboring T21, where every chromosome has reduced interactions with other chromosomes and increased self-interactions compared with NPCs with unaltered chromosome number.
“The nucleus of a cell is like an elevator when it’s full of people,” said lead author Hiruy Meharena. “It’s already at capacity, and then this one additional chromosome wants to come in, so every other chromosome squishes together to make space.”
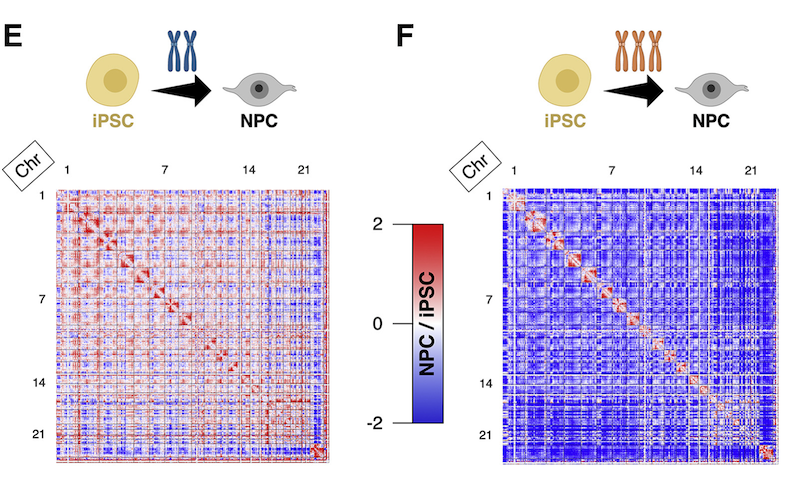
The genome is organized into two compartments within the nucleus: the active (A) and inactive (B) compartments. The A compartment is localized toward the nuclear core, and the inactive (B) compartment is localized toward the periphery on the nuclear lamina — a structure near the inner nuclear membrane. This peripheral DNA is wound up into chromatin that is enriched for a mark called histone modification H3K9me3 that indicates the repression of gene activity. By examining the localization of H3K9me3 to interrogate the consequences of T21 on genome organization, Meharena and colleagues found that while the overall architecture of the nuclear envelope and heterochromatin organization in T21 iPSCs remains unchanged, they observed increased number of H3K9me3 aggregates specifically within the nuclei of T21 NPCs and not iPSCs.
Senolytics ameliorate the T21-induced characteristics in neural progenitor cells
Chromosomal introversion and disruption of the nuclear lamina are key features of senescence. When Meharena and colleagues analyzed the nuclear architecture of three different individuals with Down syndrome, they revealed that T21 NPCs exhibit architectural changes within the nucleus that appear similar to those observed in senescent cells. Additionally, analysis of gene activity confirmed that differentially activated genes identified in NPCs harboring T21 are highly correlated with those identified in senescent cells.
“It’s a very similar phenomenon to what’s observed in senescence,” said Meharena. “This suggests that excessive senescence in the developing brain induced by the third copy of chromosome 21 could be a key reason for the neurodevelopmental abnormalities seen in Down syndrome.”
Recent studies have established the promising therapeutic potential of targeting senescent cells. Senolytic drugs not only eliminate senescent cells but also restore key cellular and molecular hallmarks associated with senescence. Meharena and colleagues found that treating T21 NPCs with the senolytic drug combination of dasatinib plus quercetin ameliorated the senescence-associated genome-wide gene activity and chromatin architecture disruptions. Moreover, treatment with senolytics also restored the migratory and proliferative capacity in T21 NPCs, which are hallmark dysfunctions of the developing brain in individuals with Down syndrome.
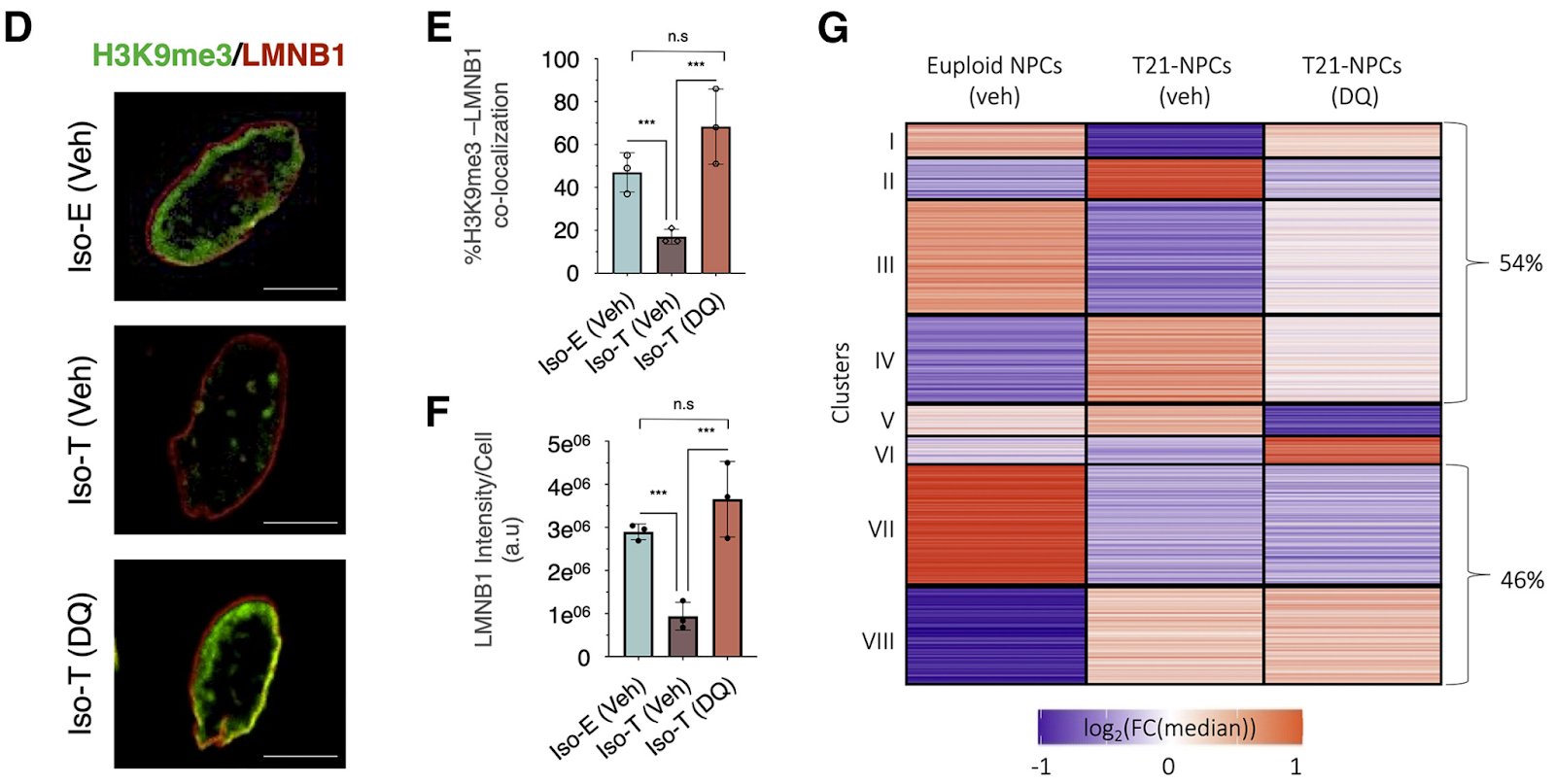
(Meharena et al., 2022 | Cell Stem Cell) Senolytic drugs alleviate the dysfunctions induced by trisomy 21 in neural progenitor cells. Five days of dasatinib plus quercetin (DQ) treatment in trisomy 21 progenitor cells (Iso-T) restored the heterochromatin distribution, as measured by the co-localization of H3K9me3 and LMNB1 (E) and total LMNB1 levels (F) to similar levels observed in the non-T21 NPCs (Iso-E). Additionally, senolytic treatment in T21 NPCs reverted the gene regulation pattern of several key clusters to that of non-T21 (euploid) NPCs (G).
It is important to note that given the way that senolytics work, which is by eliminating senescent cells, that the cells treated likely did not undergo changes that reverted them back into a healthy state. Rather, the problematic senescence-like cells were likely eliminated, which improves the state of the remaining population of NPCs.
But, in an interview with STAT, Meharena emphasized that the idea of using senolytics was just a proof of concept. “It wasn’t to show senolytics as a therapeutic intervention,” he said. “It’s still far too early for that.” Rather, he hopes that by identifying trisomy-induced senescence as a potential driver of the neurodevelopmental abnormalities seen in Down syndrome, his work might inspire new areas of research into future treatments.
Can anti-aging drugs treat Down syndrome?
While the new research points to some new explanations as to the causes of Down syndrome characteristics in brain cells, it raises many questions as to how the disease unfolds. For example, the stem cells from these Down syndrome patients did not exhibit the same disrupted nuclear organization seen in NPCs. According to Meharena, “Whatever disruption is happening is kicking in at the neural progenitor state,” he said.
This begs the question: what’s going on in these stem cells that is holding the proper nuclear architecture in place? “If we can learn why stem cells seem to be able to incorporate the additional chromosome without any major issues, maybe we can apply that to therapeutic interventions,” said Meharena. “At the very least, we hope it opens up new avenues for how we look at Down syndrome — that there seems to be this whole other element that plays on a different timeline that we really need to explore more.”

