Neuroscientists Revive Memory Deficits and Brain Cell Loss in Diabetic Rats
Nicotinamide mononucleotide (NMN) intervention protects neurons and memory from diabetes-induced brain degeneration in rats.
The brains of many people with diabetes often undergo changes that are hallmarks of Alzheimer’s disease and dementia. Diabetes patients with cognitive dysfunction—the loss of intellectual functions like thinking, remembering, and reasoning severe enough to interfere with day-to-day living—typically have damage to nerve cells, supportive brain cells, and blood vessels in the brain, causing them to live in a cognitive fog.
NMN Promotes Neuronal Survival and Memory Function
Chandrasekaran and colleagues recently published a research article in the International Journal of Molecular Sciences indicating nicotinamide mononucleotide (NMN) promoted neuronal protection, survival, and regeneration resulting in the preservation of cognitive functions in diabetic rats. NMN protected neurons in the brain region involved in forming new memories called the hippocampus, which preserved memory function. These findings suggest that NMN may provide therapeutic intervention for brain damage and dysfunction in diabetic patients.
Diabetes lowers the levels of a key molecule linked with aging and longevity called nicotinamide adenine dinucleotide (NAD+). This vital compound is critical for the functioning of many molecular machines that catalyze reactions (i.e., enzymes) and the cell’s energy generator, the mitochondria. Therapeutic interventions with compounds that can be converted to NAD+, such as NMN and nicotinamide riboside (NR), can replenish cellular NAD+ levels and have drawn significant attention in the field of aging, obesity, and diabetes.
NMN administration has been shown to alleviate age-associated deterioration in the liver, adipose tissue (fat), muscle, pancreas, kidney, retina, and central nervous system of animals. Interventions with NMN have been shown to effectively increase NAD+ levels in various parts of the body, including the brain, suggesting that it crosses the blood-brain barrier. This has triggered intense interest in boosting NAD+ levels to prevent or revert brain degeneration and subsequent cognitive dysfunction in diabetic patients.
The research team from the University of Maryland tested if NMN administration prevented NAD+ deficiency, brain damage, and cognitive dysfunction in diabetic rats. These animals had decreased levels of NAD+ in the brain compared to those without diabetes, and when the researchers administered NMN beneath their skin, the levels of NAD+ in the brain increased.
Similarly, the researchers observed that diabetic rats had decreased levels of a protein linked to aging and longevity called SIRT1. But the levels of SIRT1, which is central to the regulation of both mitochondrial function and neuronal preservation, recovered to the same levels as non-diabetic rats upon treatment with NMN.
The mitochondria in the brain region linked to the formation of new memories, the hippocampus, of diabetic rats were worse off structurally and functionally. Assessment of mitochondrial function showed that the hippocampal neuronal mitochondria were energetically stressed and had an increased workload in diabetic compared to non-diabetic mice. But the administration of NMN relieved both the energetic stress and workload of mitochondria in the hippocampus of diabetic mice.
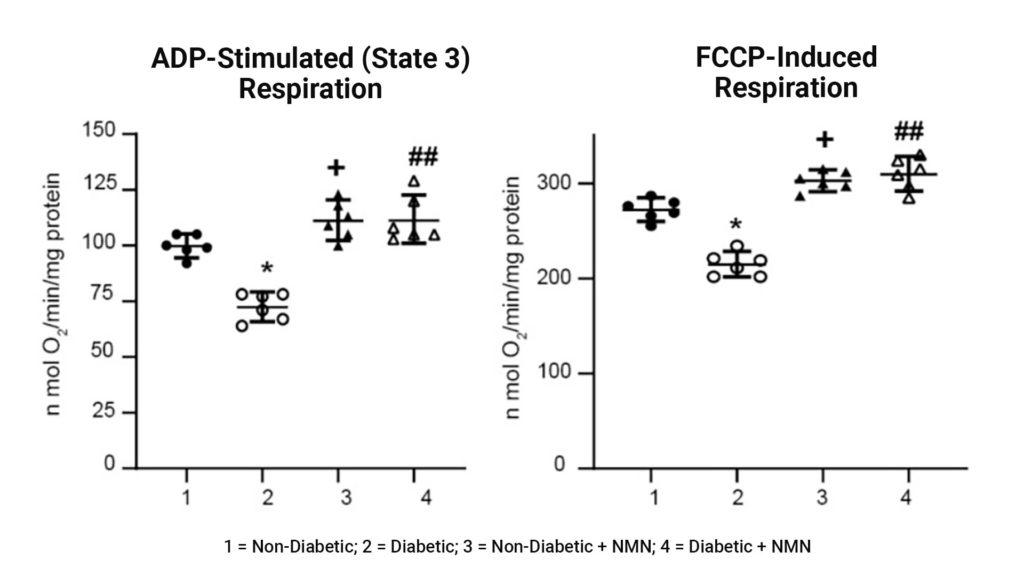
Impaired mitochondrial respiration in Diabetic rats was prevented by NMN treatment. Assessment of mitochondrial function showed that both the amount of extra energy that can be produced in case of a sudden increase in demand (ADP-stimulated respiration) and maximum workload (FCCP-induced respiration) were decreased in hippocampal mitochondria from the diabetic compared to the non-diabetic rats. The administration of NMN increased both spare reserve capacity and maximum workload.
The investigators found that the volume and the number of neurons in a region of the hippocampus called CA1 that is involved in learning and memory significantly declined in the diabetic rats compared to non-diabetic rats. NMN prevented the diabetes-induced loss of hippocampal neurons, paralleling the activation of the SIRT1 pathway and the preservation of mitochondrial function.
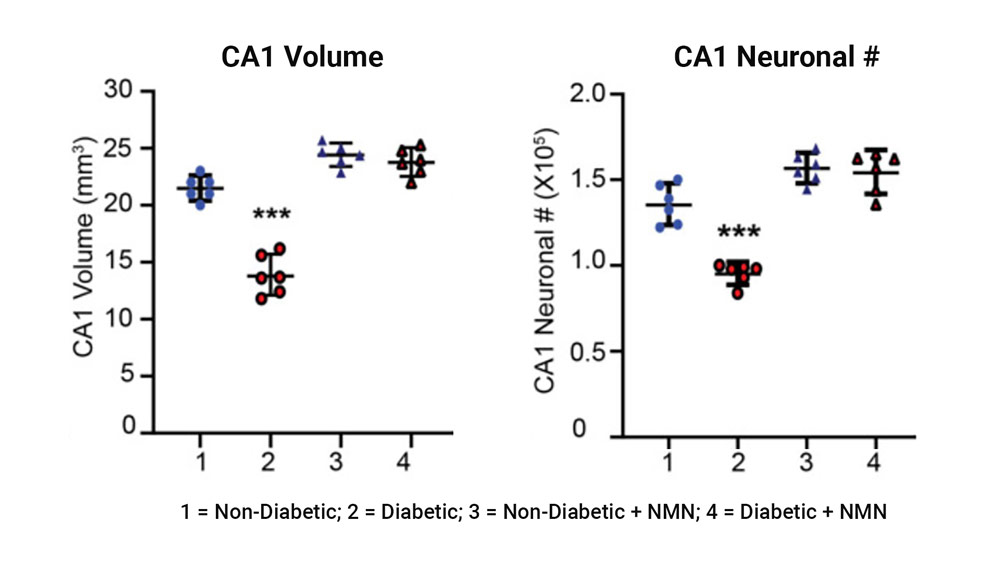
Diabetes-induced loss of hippocampal volume and neuronal counts in CA1 is prevented by NMN-treatment. The left plot shows the comparison of the CA1 hippocampal regional volume between non-diabetic and diabetic rats showed a significant difference. The right plot shows the comparison of the CA1 hippocampal neuronal number (#) between non-diabetic and diabetic rats showed a significant difference.
Since the CA1 region of the hippocampus is involved in learning and memory, particularly the ability to spatial learning and memory, the researchers looked at whether diabetes disrupted these cognitive abilities and if NMN could rescue them. Spatial memory refers to the ability to memorize and recall locations necessary for navigating spaces, and can be tested using the Y maze.
Consisting of three arms that are arranged in the shape of a capital Y, the Y maze measures the willingness of rodents to explore new environments because they typically prefer to investigate a new arm of the maze rather than returning to one that was previously visited. The maze is ideal and effective for studying various facets of spatial memory, especially impairments to it. Rodents with impairments to spatial memory will not enter and spend as much time exploring the new arm compared to unimpaired rodents, which indicates that they cannot distinguish or remember the different arms of the maze.
Using the Y-maze to assess spatial memory, the investigators saw that the diabetic rats entered and spent about half the amount exploring the new arm of the maze compared to non-diabetic rats. However, this deficit in spatial learning and memory seen in diabetic rats was reversed with NMN administration.
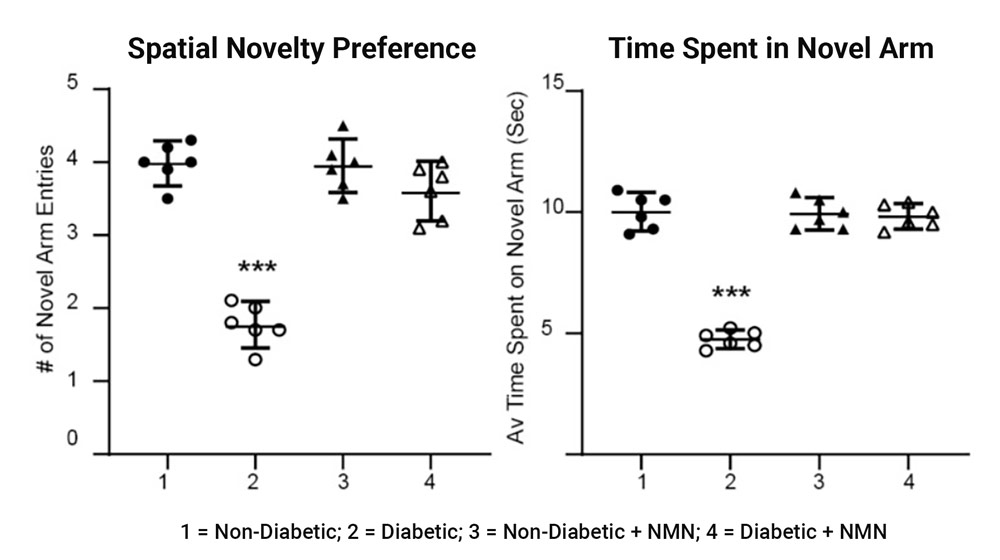
Impaired memory in diabetic rats was prevented by NMN treatment. Using a Y maze, the investigators determined differences in memory between the four groups of rats. The maze assesses rapidly acquired short-term spatial memory and relies on the fact that normal rats prefer novel over familiar spatial environments.
The findings in this study indicate that NMN prevented the diabetes-induced decrease in NAD+ levels, normalized SIRT1 protein levels, and improved downstream activities such as mitochondrial function, which are mediated by NAD+, to protect neurons from diabetes-induced degeneration of neurons. This study suggests that medications that prevent NAD+ depletion induced by diabetes and molecules that activate SIRT1 may be therapeutic for diabetic deterioration of the brain.
“Our results indicate that NMN increased brain NAD+, activated the SIRT1 pathway, preserved mitochondrial function, prevented neuronal loss, and preserved cognition in diabetic rats,” said the investigators in the study. “The study suggests that medications that prevent NAD+ depletion induced by diabetes mellitus and molecules that activate SIRT1 may provide a therapeutic intervention for diabetic neurodegeneration in the central nervous system.”

