New Harvard-Mayo-Cedars-Sinai Joint Study Confirms Safety of Anti-Aging Senolytic Treatment
The senolytics dasatinib and quercetin (DQ) are safe and tolerable for older adults with cognitive and motor impairments, but do they improve memory and movement?
Highlights
- Senolytics are an exciting anti-aging technology that may potentially treat multiple age-related diseases, including dementia.
- The senolytics DQ were safe and led to no serious side effects in individuals at risk for Alzheimer’s.
- DQ reduced an inflammatory marker that was correlated with improved memory.
Alzheimer’s disease (AD) robs individuals not only of their memories but also of their balance, walking speed, and strength. But what if combating this catastrophic condition lay not in profitable drugs, but in targeting one of aging’s root causes? This is explored in a new study by researchers from Harvard Medical School, the Mayo Clinic, Cedars-Sinai Medical Center, Beth Israel Deaconess Medical Center, and the Hinda and Arthur Marcus Institute for Aging Research.
What Are Senolytics?
As we age, some of our cells enter a state called senescence. These ‘senescent cells’ release a cocktail of inflammatory molecules and create a toxic environment that can damage surrounding healthy cells. As they accumulate over time, senescent cells contribute to a wide range of age-related diseases, including neurodegenerative conditions like AD.
For years, scientists have investigated how to clear these problematic senescent cells from the body. This is where senolytics come into play. These compounds can selectively eliminate senescent cells, thereby reducing the harmful molecules they emit, potentially mitigating the effects of biological aging. The new Harvard study focuses on two such senolytic agents: dasatinib and quercetin, often referred to as DQ.
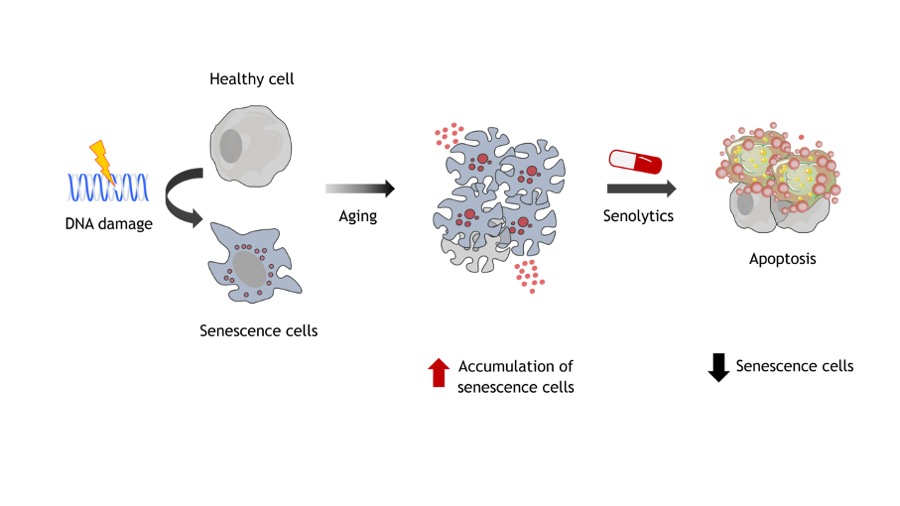
While previous research in mice has shown promising results in improving both physical and cognitive function, the real question has always been: can these findings translate to humans, particularly those at risk for AD?
The Who and What of the Study
The study was a pilot investigation, meaning it was a small-scale, preliminary study designed to assess the feasibility, safety, and initial effects of DQ in a human population. The researchers focused on older adults who were at risk for AD, specifically those exhibiting mild cognitive impairment (MCI) and slow gait speed – two common indicators of increased risk. This particular group is highly relevant because they often suffer from falls, frailty, and a loss of independent physical function, making preventive interventions critically important.
Participants in this study received a specific regimen of senolytic agents: 100 mg of dasatinib and 1,250 mg of quercetin. Crucially, this wasn’t a daily dose. Instead, the compounds were administered for just two days every two weeks, over a total period of 12 weeks. This intermittent dosing strategy is based on preclinical studies suggesting that senolytics don’t need to be continuously present to be effective. Senescent cells, once targeted, take time to re-accumulate, allowing for a pulsed treatment approach that could minimize potential side effects.
Safe: No Serious Side Effects
While the study was small, involving only 12 participants, the results are meant to offer compelling hints. First and foremost, the treatment appeared to be both feasible and safe. There were no serious adverse events reported, which is a critical finding for any new therapeutic approach. This suggests that intermittent DQ treatment could be well-tolerated in this vulnerable population.
Cognitive Improvements
Beyond safety, the study also looked for preliminary signs of efficacy. One key measure was the Montreal Cognitive Assessment (MoCA) score, a widely used tool to screen for cognitive impairment. While the average MoCA scores across all participants didn’t show a statistically significant increase, a more granular look revealed something intriguing: in participants with the lowest baseline MoCA scores, there was a significant increase of 2.0 points. This suggests that those who were most cognitively impaired at the start of the study might benefit the most from the intervention, a finding that warrants further investigation.
Reduced Senescent Markers Correlate with Better Memory
Another crucial aspect of the study involved tracking biomarkers related to cellular senescence. The researchers specifically measured levels of tumor necrosis factor-alpha (TNF-α), a prominent molecule secreted by senescent cells. While the overall decrease in TNF-α levels wasn’t statistically significant across all participants, a vital correlation emerged: reductions in TNF-α were significantly and inversely correlated with increases in MoCA scores.
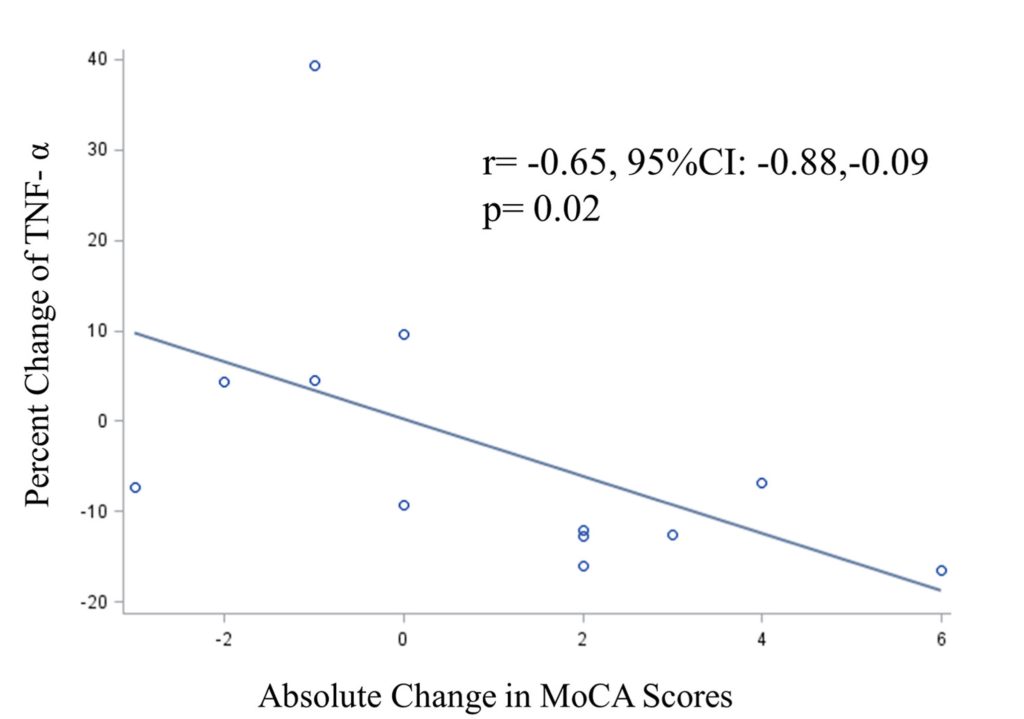
In simpler terms, the more a participant’s TNF-α levels decreased, the more their cognitive scores improved. This link provides a biological mechanism by which DQ might be exerting its cognitive benefits – by reducing the harmful inflammatory environment created by senescent cells.
The Geroscience Hypothesis in Action
This study provides valuable human data supporting the geroscience hypothesis, an emerging field that seeks to understand the fundamental mechanisms of aging and how they contribute to age-related diseases. The idea is that by targeting these basic aging processes, we can prevent or treat a wide range of conditions, rather than just focusing on individual diseases. Senolytics, by clearing out senescent cells, are a prime example of a geroscience-based intervention.
Previous research, primarily in animal models, has shown that senolytic therapies can extend health longevity and improve various age-related conditions, including physical function, vascular stiffness, fatty liver, osteoporosis, and even neurodegenerative changes in models of AD. This pilot study, though small, takes a crucial step in translating these promising findings from the lab to human patients, particularly those at a critical juncture in their risk for AD.
The Road to Confirmation
It’s important to approach these findings with cautious optimism. As a pilot study, its primary goal was to assess feasibility and safety, and to provide preliminary data to inform larger, more definitive trials. The small sample size (n=12) and the absence of a control group mean that the results, while encouraging, cannot be considered conclusive. Without a control group, it’s difficult to definitively attribute the observed improvements solely to the DQ treatment, as other factors could be at play.
Participants: Older adults (≥ 65 years) with slow gait speed and mild cognitive impairment
Dosage: 100 mg of dasatinib and 1,250 mg of quercetin taken for two consecutive days every two weeks for 12 weeks (six cycles)

