New Johns Hopkins Study: Senescence Links Dementia to Osteoporosis
Osteoporosis — age-related bone loss — is prevented by removing (senescent) cells that promote the accumulation of Alzheimer’s proteins in old mice.
Highlights
- Senescent fat cells in bone marrow secrete a molecule called SAP that plays a role in the formation of Alzheimer’s plaques.
- Removing senescent cells with senolytics prevents age-related bone loss.
- Considering previous studies, senolytics can potentially target both Alzheimer’s dementia and osteoporosis simultaneously.
For decades, Alzheimer’s disease (AD) has primarily been viewed as a brain disorder, characterized by the accumulation of amyloid-beta proteins. Yet, a new study published in Nature Aging by researchers from Johns Hopkins University is now expanding this brain-centric view, showing that amyloid-beta exists outside of the brain.
“While brain amyloid has been extensively studied for its role in memory loss and neurodegeneration, far less is known about amyloid elsewhere in the body. In fact, almost nothing is known about whether amyloid forms in the skeleton or how it might contribute to age-related bone loss,” said Mei Wan, PhD, one of the authors of the new study.
Cellular Senescence & Amyloid-Beta
Bone marrow contains a diverse range of cell types, including stem cells that give rise to our immune cells. Less well-known are the fat cells that reside in our bone marrow. These fat cells not only increase in number as we age, but they can also enter a state of cellular senescence.
Cellular Senescence
What is cellular senescence? Senescence occurs in cells that have encountered molecular or environmental stress. For example, a skin cell that encounters too much ultraviolet (UV) radiation can enter a state of senescence, becoming a senescent cell. In a similar fashion, when the brain encounters too much DNA damage, it can trigger a neuron to become a senescent cell. Throughout the body, as molecular and environmental stressors accumulate with age, so do senescent cells.
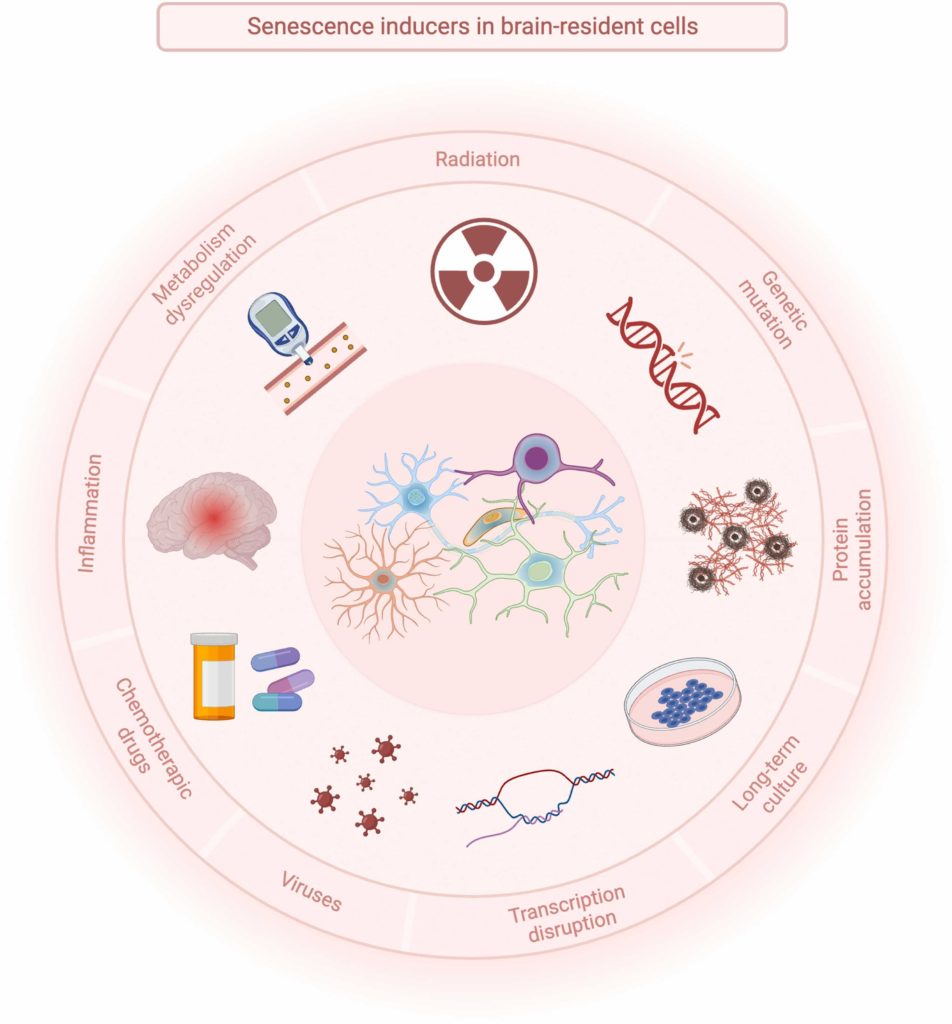
How do senescent cells contribute to age-related diseases? Senescent cells secrete a wide variety of molecules, including pro-inflammatory and organ-damaging molecules. These molecules support chronic inflammation and disease. They are collectively known as the senescence-associated secretory phenotype (SASP). As revealed by the Johns Hopkins researchers, scientists are still discovering new SASP factors and how they contribute to biological aging.
Amyloid-Beta Surrounds Senescent Cells in Bone
The new study focused on senescent cells, specifically senescent fat cells in the bone marrow of mice. The Johns Hopkins researchers found a significant accumulation of senescent fat cells in the bone marrow of both old mice and mouse models of AD. And what’s more, the senescent fat cells were surrounded by amyloid-beta deposits, the very same protein aggregates found in AD patient brains.
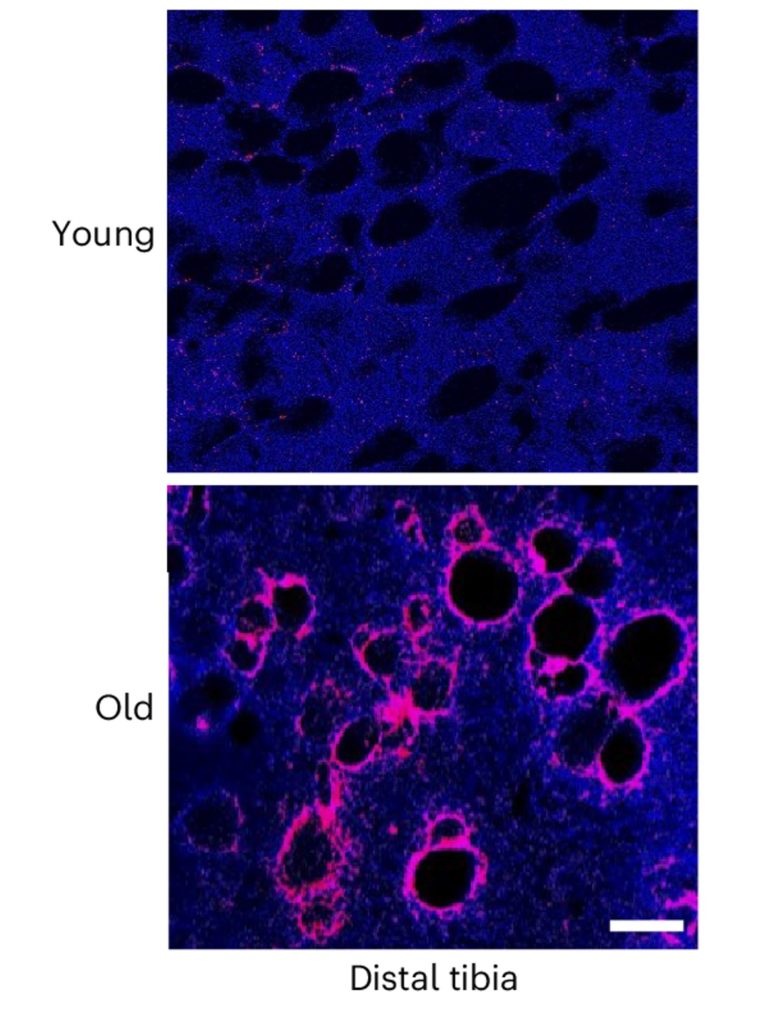
The researchers also pinpointed a specific SASP factor secreted by the senescent fat cells called SAP (serum amyloid P component). SAP was first discovered in amyloid-beta deposits and is known to stabilize amyloid-beta fibrils. While not full-fledged plaques, fibrils are an aggregated form of beta-amyloid proteins.
Preventing Bone Loss with Senolytics
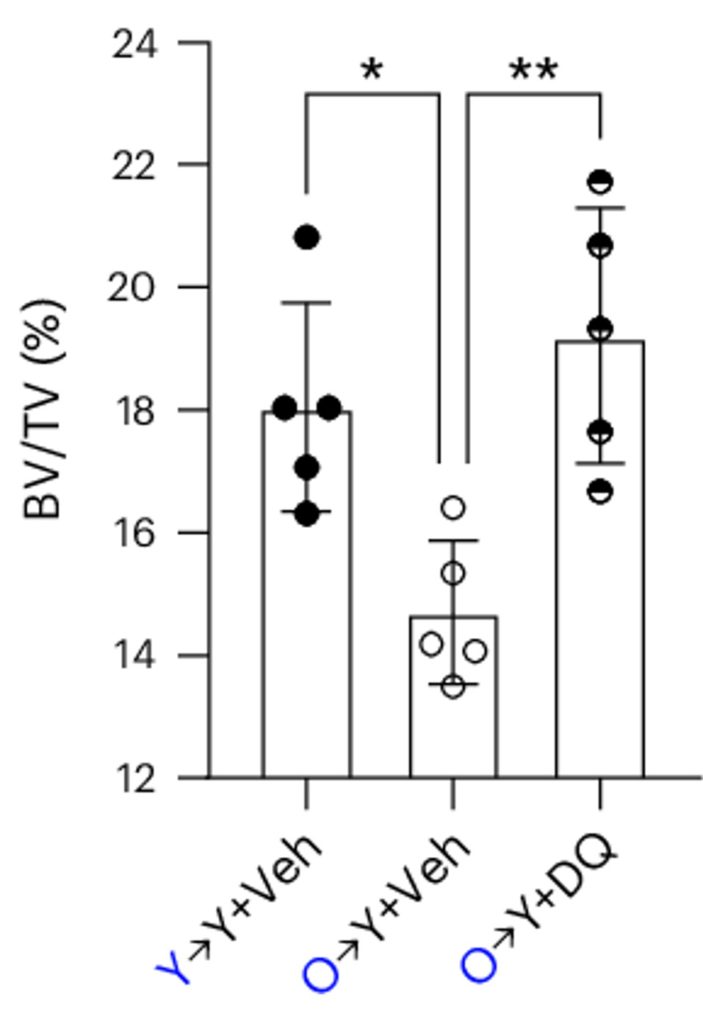
Since both old mice and mouse models for AD exhibit measurable bone loss, the researchers sought to determine if the senescent fat cells contribute. To do so, they transplanted bone marrow fat cells from old mice into young mice. This resulted in the young mice developing bone loss, a clear indication of the direct role of these aging fat cells. Crucially, treating these mice with senolytics — compounds that selectively eliminate senescent cells — prevented the bone loss.
Moreover, depleting SAP in old mice not only abolished marrow amyloid deposition but also rescued their low bone mass. SAP depletion was accomplished by using the pharmaceutical drug miridesap, which specifically targets SAP for the treatment of amyloid-beta aggregation.
How Senescent Cells Lead to Bone Loss
Further experiments revealed that the SAP secreted from senescent fat cells in bone marrow, when combined with amyloid-beta, has a detrimental effect on bone remodeling. It encourages the formation of osteoclasts (cells that break down bone) while inhibiting osteoblasts (cells that build new bone). This imbalance leads to bone loss, a condition often seen in aging.
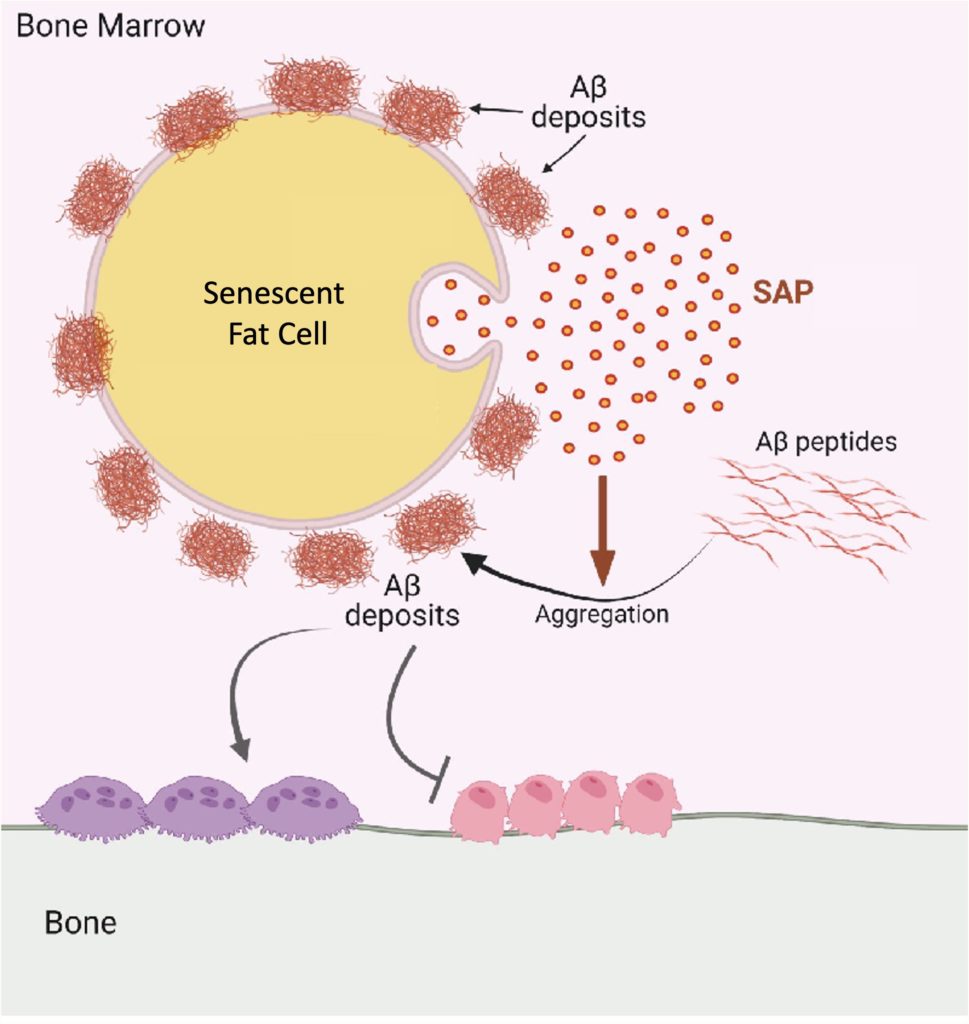
“Our study is what we believe to be the first to show that harmful amyloid fibers (Aβ fibrils) build up in the bone marrow of aged mice. We also found that fat cells in the bone marrow release a protein called SAP/PTX2, which plays a major role in triggering this amyloid buildup and damaging bone. These findings uncover a new connection between bone loss and dementia risk and may open the door to new research on how protecting bone health could also help protect brain function,” said Dr. Wan.
Targeting Alzheimer’s and Osteoporosis with Senolytics
Intriguingly, low bone mineral density is associated with a higher risk of developing AD. Moreover, bone loss, which is more frequent in AD patients, occurs early in the progression of AD. These findings suggest that both age-related diseases can potentially be targeted by the same intervention. While the link between AD and osteoporosis derives primarily from the presence of amyloid-beta accumulation, what both also have in common is cellular senescence.
A clinical trial has already shown that the senolytic combo dasatinib and quercetin (DQ) is safe and tolerable for early-stage Alzheimer’s patients. Future trials will confirm or deny whether this senolytic combo can slow the progression of AD, but the outlook seems hopeful. Furthermore, in a phase 2 clinical trial, DQ was shown to increase a marker for bone formation in older women, suggesting that DQ may play a role in mitigating osteoporosis.
Future clinical trials testing the effects of DQ and other senolytics, such as fisetin, on AD and osteoporosis may prove that senolytics are an effective treatment for both age-related diseases. With that being said, senolytics could also potentially play a preventative role against AD and osteoporosis. That is, while the damage done by senescent cells and the SASP may be irreversible, removing senescent cells before they cause the damage may prove beneficial.
Model: 4-month-old (young) mice transplanted with bone marrow fat cells from 24-month-old (old) mice
Dosage: Injection of 5 mg/kg of dasatinib and 50 mg/kg of quercetin

