New Pilot Human Trial Supports Probiotics Improving Mental Well-Being in the Elderly
Oral supplementation with the probiotic Lactiplantibacillus plantarum improves scores on an assessment of quality of life and reduces gut inflammation in older adults.
Highlights
- Oral supplementation with Lactiplantibacillus plantaris (L. plantaris) for 12 weeks significantly improved scores on a subjective assessment of quality of life.
- Older participants who took the probiotic exhibited significantly lower fecal levels of calprotectin, a protein strongly associated with gut inflammation.
- Analysis of the gut bacterial composition found that the probiotic increased the abundance of beneficial gut bacteria.
The gut-brain axis is a bidirectional communication network linking the nervous system to the gut. It enables constant, two-way signaling via neural, hormonal, and immune pathways, allowing gut microbes to influence mood and cognition, while the brain regulates intestinal function.
Gut dysbiosis, a functional imbalance in the gut microbial ecosystem, has emerged as a key factor influencing cellular aging and age-related diseases across the human lifespan, through its influence on the gut-brain axis. More specifically, gut dysbiosis compromises gut barrier integrity, leading to increased permeability (known as a “leaky” gut), which exposes systemic blood circulation to pathogens and toxic metabolites from the gut. These pathogens and metabolites contribute to low-grade, systemic inflammation, tissue damage (including damage to neural tissue), and other processes related to aging. Notably, in relation to the nervous system, gut dysbiosis has also been linked to the progression of neurodegenerative changes during aging through its influence on the gut-brain axis.
To counteract gut dysbiosis and its potential influence on neurodegeneration in older adults, gut microbe-targeting interventions have been studied, particularly those aligned with eating a fiber-rich diet. Probiotics, live microorganisms that confer beneficial effects when consumed in adequate amounts, have also garnered growing attention for their potential to influence a broad range of physiological processes, such as the amelioration of neurodegeneration-related symptoms. However, there is still a lack of human trial evidence supporting the use of probiotics as a preventative agent against neurodegenerative processes during aging.
Now, as published in NPJ Aging, Mytych and colleagues from Olimp Laboratories in Poland unveil data from a pilot human trial that employed a randomized, double-blind, placebo-controlled method (the highest standard of assessing causation). In the trial, the researchers tested an oral probiotic, a strain of L. plantaris (OL3246), in older adults and found that 12 weeks of supplementation improved scores on subjective assessments of quality of life and mood. Moreover, fecal samples from participants who received the probiotic contained lower levels of calprotectin, a protein strongly associated with gut inflammation, than non-treated participants at the end of the supplementation period. Also, an analysis of gut microbes showed that supplemented participants had increased levels of beneficial gut bacterial species, such as Fecalibacterium prausnitzii, a species widely recognized for having anti-inflammatory properties. These findings support the notion that supplementing with the probiotic L. plantaris improves aspects of the gut-brain axis, thereby enhancing feelings of well-being and mood with advanced age.
The Probiotic L. plantaris Enhances Well-Being and Lowers Gut Inflammation
For their study, Mytych and colleagues split approximately 30 participants into two groups: one receiving the probiotic supplement and the other receiving a placebo for 12 weeks. The participants were healthy older adults between 55 and 85 years old.
Because a gradual decline in perceived quality of life, as well as mood-related disturbances, frequently accompanies aging, Mytych and colleagues administered two assessments of well-being and mood. One of the tests, the SF-36, is a 36-item self-reported questionnaire that measures quality of life across physical and mental health dimensions. The other test, the Beck Depression Inventory, is a 21-item self-report questionnaire, measuring the presence and severity of depressive symptoms.
Interestingly, following 12 weeks of supplementation with the probiotic, the researchers observed improvements for both of these assessments. These findings suggest that the probiotic supplement improves self-reported dimensions of quality of life and mood. However, since the questionnaires relied on subjective reports, future human trials should use more detailed evaluations of quality of life and mood, such as one-on-one interactions between participants and clinicians trained to gauge these effects.
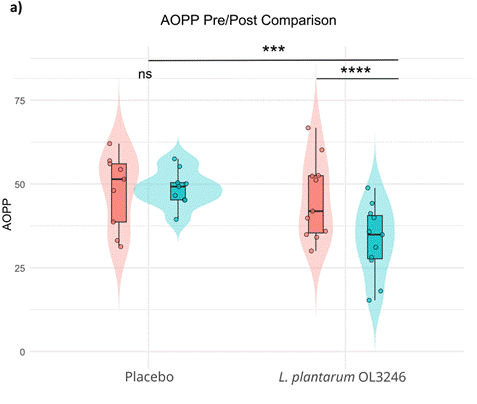
One mediator of inflammation that accumulates in the blood with age, advanced oxidation protein products (AOPPs), can contribute to molecular damage, such as protein misfolding and DNA damage. These harmful molecular processes are recognized as contributors to neurodegenerative diseases and reduced feelings of well-being.
Given what research has uncovered about AOPPs’ link to neurodegeneration and reduced well-being, Mytych and colleagues measured the effects of probiotic supplementation on AOPP levels in the blood. Interestingly, they found that after supplementation with the probiotic, AOPP levels dropped significantly. This result supports the notion that the probiotic has positive effects on feelings of well-being and mood, in part, by lowering circulating AOPP levels.
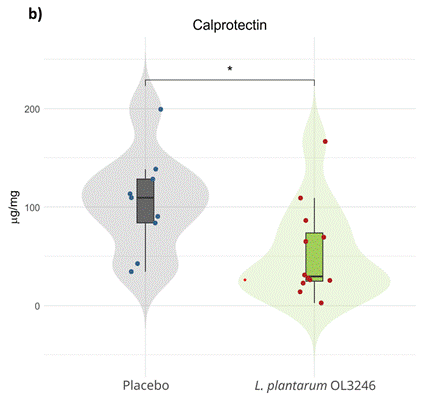
Calprotectin is a protein released by white blood cells that acts as a marker of inflammation in the gastrointestinal tract. Researchers test levels of calprotectin in stool samples to gauge intestinal inflammation levels, which can coincide with a “leaky gut.” Moreover, calprotectin levels are frequently elevated in older adults, possibly reflecting age-related increases in gut-derived inflammation. Such gut-derived inflammation could contribute to the inflammatory cascade, which propagates from the gut to the blood, ultimately affecting the brain and contributing to neurodegeneration.
Due to gut inflammation’s possible contribution to neurodegeneration, and given calprotectin’s ability to serve as a marker of gut inflammation, Mytych and colleagues measured calprotectin levels after supplementation. Compared to the group administered a placebo, participants supplemented with the probiotic exhibited significantly lower levels of fecal calprotectin. Although levels of calprotectin were not measured before supplementation to compare with levels after supplementation, the significant difference between participants given the probiotic and those given a placebo suggests that the probiotic lowers calprotectin, and thus, gut inflammation. If future research confirms that the probiotic lowers gut inflammation, this could signify a protective effect on the gut, preserving its integrity and preventing a “leaky gut” to lower the entrance of AOPPs from the gut into circulation.
Finally, to test whether the probiotic modulates gut microbes to increase the abundance of beneficial bacterial species, which have anti-inflammatory effects, Mytych and colleagues performed an analysis of gut bacteria after probiotic supplementation. The researchers found enrichments of species known to have anti-inflammatory effects, such as Fecalibacterium prausnitzii, following probiotic supplementation. This finding supports that supplementing with the L. plantaris probiotic increases the abundance of beneficial gut bacteria to possibly lower inflammation. The presence of gut bacteria with anti-inflammatory effects could help preserve the integrity of the gut lining, preventing a “leaky gut,” to reduce AOPPs from entering circulation and ultimately wreaking havoc on the brain.
Supplementing with Probiotics for Mental Well-Being with Advanced Age
A key limitation of this pilot human trial is that it only had a small number of participants, about 30, which limits the statistical power of its findings. However, results from the trial provide what may be a promising glimpse of evidence that a strain of the probiotic L. plantaris modulates gut bacteria, increasing beneficial bacteria with anti-inflammatory properties. The study also provides evidence that the probiotic lowers gut inflammation, which may positively influence the gut-brain axis and help explain why participants who took the probiotic reported enhanced well-being and mood. Future trials with more participants and more detailed assessments of well-being and mood will be necessary to confirm these findings.
For those who want to hedge their bet on the evidence from this pilot trial, strains of the probiotic L. plantaris are available in capsule form for between $20 and $40 for a month’s supply. The particular strain of this probiotic used in the trial, OL3246, is not specifically available currently. Some sources state that positive effects from L. plantaris may be strain-specific, so whether other strains of the probiotic species used in this human trial would confer the same effects remains unclear. Also, assessing the effects of other strains will require future human trials.
Furthermore, Mytych and colleagues did not provide any reasoning behind why they chose the OL3246 strain they used for their study. Without knowing why they chose this particular strain, it remains difficult to discern what differentiates it from other strains. It could be that all strains have some degree of efficacy in lowering gut and systemic inflammation. In that regard, another human trial showed another strain of L. plantaris conferred a statistical trend toward lowering systemic inflammation in older adults. Hence, future research should also compare strains of particular probiotic species and determine which ones have the greatest effects in alleviating systemic inflammation, improving facets of the gut-brain axis, and enhancing mental well-being.
Model: 26 healthy adults between the ages of 55 and 85
Dosage: Oral Lactiplantibacillus plantaris OL3246 (370 mg capsule containing 7 × 109 CFU per capsule) twice daily for 12 weeks

