New Scientific Insights on Slowing Aging and Promoting Longevity from Global Experts
Top aging scientists propose two new hallmarks of aging: extracellular matrix changes (alterations to the networks of molecules that support cells) and psychosocial isolation (a lack of meaningful social interaction).
Highlights
- Top aging researchers have added two new hallmarks of aging—extracellular matrix changes and psychosocial isolation—making a total of 14 hallmarks of aging.
- The researchers also propose therapeutically suppressing genes that drive aging (gerogenes) and activating genes that counteract aging (gerosuppressors).
- Furthermore, the researchers suggest initiating clinical care called geromedicine that applies aging intervention measures based on medical history and genetic evaluations.
In 2013, the inception of nine hallmarks of aging was a crucial conceptual development in the field of aging research. These hallmarks of aging included biological phenomena that met three stringent criteria: their manifestation being associated with aging over time, their experimental accentuation accelerating the aging processes, and their suppression decelerating aging. The listed hallmarks of aging from 2013 included things like dysfunction of the cell’s powerhouse (mitochondrial dysfunction) and the shortening of protective ends of chromosomes (telomere attrition). Then, based on the three noted criteria and new research developments, in early 2023, aging researchers listed three more hallmarks of aging, making a total of 12.
Identifying these hallmarks of aging sparked a significant expansion in public awareness of aging research. Moreover, some biotech companies have subsequently developed therapeutics based on these hallmarks of aging, claiming to target one, several, or all of them at once.
Now, López-Otín and colleagues from the University of Nebrija in Spain have added two more hallmarks of aging to this repertoire, as published in Cell. With expanding knowledge of aging based on new research, the researchers have lamented that the 12 hallmarks proposed in 2023 are outdated and have thus added extracellular matrix changes and psychosocial isolation to the list. This new development, making the currently listed hallmarks of aging total 14, could pave the way for tailoring aging interventions that target these two new hallmarks of aging.
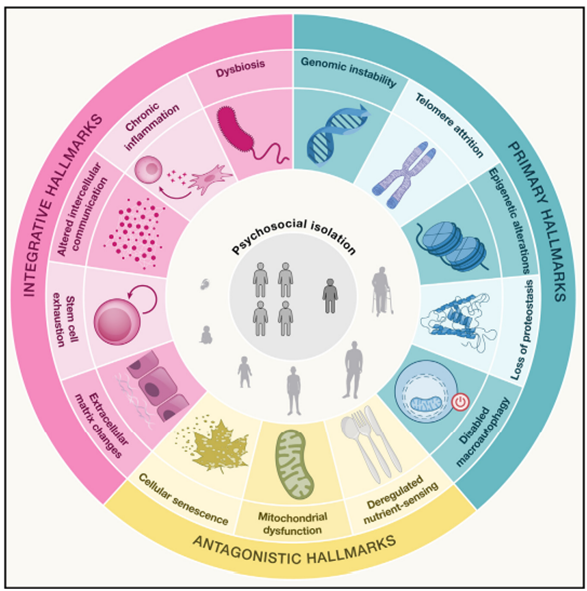
Delving Into Extracellular Matrix Changes and Psychosocial Isolation
As for why changes to the extracellular matrix, a network of molecules that surrounds and supports cells, have been added, a progressive reduction in extracellular matrix elasticity and overall functional capacity accompanies aging for cells throughout the body. Moreover, genetically engineering mice that express a gene for an enzyme called Has2 from long-lived naked mole rats extends lifespan. Inserting the naked mole rat gene for Has2 into mice drives the production of an extracellular matrix component, which exerts protective effects on cells. These findings suggest that the extracellular matrix plays a key role in longevity and that its age-related changes serve as a hallmark of aging.
The other hallmark of aging added to the list, psychosocial isolation, has been neglected, according to the researchers, due to an excessive focus on cellular and molecular pathways. Reasons behind adding this hallmark of aging come from evidence from animal studies and human trials that psychosocial adaptation—an individual’s ability to adjust to changing life circumstances—is an essential feature of organismal health during aging and that its deterioration leads to psychosocial isolation. Furthermore, according to López-Otín and colleagues, psychosocial isolation fulfills the three criteria of a hallmark of aging.
Along those lines, old age is often accompanied by exclusion from societal activities. This occurrence coincides with reduced fitness, declining motor coordination, and memory loss. The resulting circumstance entails enfeeblement of social and emotional bonds as well as psychological consequences like anxiety, depression, loneliness, and disrupted sleep patterns. The psychological consequences of psychosocial isolation can be pathogenic, likely due to activating stress responses. In mice, social interaction between younger and older mice increases the longevity of the older mice, suggesting that social support can allay the detrimental effects of age-related psychosocial isolation.
Limitations Associated with the Hallmarks of Aging Model
While the hallmarks of aging may be useful therapeutic targets, López-Otín and colleagues lament that their application for aging interventions is limited. Such limitations come from their interconnectedness and the difficulty of separating them from one another.
For example, using molecules called senolytics to eliminate dysfunctional cells that accumulate with age (senescent cells) suppresses inflammation and improves intercellular communication. Senescent cell buildup, inflammation, and altered intercellular communication are all hallmarks of aging, so the example of senolytics highlights how the hallmarks of aging may not necessarily be separate pathways but instead biological entry points to experimentally manipulate processes of aging.
Gerogenes Contribute to Aging and Gerosuppressors Counteract Aging
To get around the lack of clarity arising from the interconnectedness of the hallmarks of aging, López-Otín and colleagues proposed a new way to confront aging with potentially more precision. In that sense, they identified certain genes that research suggests contribute to aging when overactivated, called gerogenes. In a similar vein, the researchers also identified some genes that actively counteract aspects of aging called gerosuppressors.
According to López-Otín and colleagues, gerogenes and gerosuppressors have not evolved with the purpose of regulating processes of aging. Instead, they support an array of molecular functions that contribute to aging-related processes from their activation over time. Hence, gerogenes could hasten aging when overactivated, whereas gerosuppressor activation may counteract certain processes of aging.
An example of a gerogene is DBI, which may show increased activity with age, increasing the likelihood of cardiovascular problems. As such, a drug already approved by the FDA called resmetirom inhibits DBI activation and could help lower the activation of this gerogene. As this example illustrates, some drugs that have already been developed could help suppress certain identified gerogenes.
As an example of a gerosuppressor gene, ɑ-klotho is associated with human longevity, and this association has been shown when the protein that it codes for is high in blood plasma. Interestingly, the combination of two senolytics, dasatinib and quercetin, has been shown to increase levels of the ɑ-Klotho protein in the blood of patients with lung tissue scarring (pulmonary fibrosis). Hence, the example of dasatinib and quercetin serves as a possible therapeutic option to increase the activation of certain gerosuppressors, in this case ɑ-klotho.
Furthermore, several gerogene and gerosuppressor modulating drugs are under investigation in human trials. These drugs are under assessment for their capabilities to target a range of distinct age-related diseases, like high blood pressure and cancer.
Geromedicine as a Means to Administer Gerogene and Gerosuppressor Modulating Therapies
If gerogene and gerosuppressor modulating drugs are shown to be effective in alleviating certain age-related conditions, it will then be important to identify who might receive them. Determining who receives what drugs could be based on geromedicine—a new field of medicine that focuses on optimizing health and preventing diseases by targeting the underlying biological processes of aging. As such, geromedicine, which incorporates patient data from medical history and genetic evaluations, among other assessments, may give way to future clinics that apply interventions targeting gerogenes and gerosuppressors. Using geromedicine in the future, clinicians may target processes of aging to prevent age-related diseases and optimize patients’ health as they age.

