New Study Shows NMN Rejuvenates Stem Cells and Mitochondria by Activating Longevity Protein
Jilin University researchers show that nicotinamide mononucleotide (NMN) reduces stem cell senescence (aging) and restores mitochondrial function in rats by increasing the activity of sirtuin 3 (Sirt3) — a longevity-associated protein.
Highlights
- NMN decreases cellular senescence – a dormant cell state that promotes aging – in stem cells and increases mitochondrial function.
- NMN’s effects on mitochondria and cellular senescence are governed by the increased activity of sirtuin 3 (Sirt3) – a guardian protein that helps regulate mitochondria.
Stem cells have garnered extreme attention for their potential applications in regenerative medicine. They possess self-renewal properties shown to contribute to tissue healing and cartilage regeneration. However, their regenerative potential is severely compromised by cellular senescence, which occurs in the later stages of stem cell growth. Therefore, finding ways to delay senescence in growing stem cells is vital to sustaining their therapeutic potential.
Now, researchers from Jilin University in China report in the International Journal of Molecular Sciences that nicotinamide mononucleotide (NMN) attenuates stem cell senescence in late-stage stem cells. Wang and colleagues show that treating old stem cells with NMN limits senescent cell burden and boosts mitochondrial function, which is key to delaying senescence. Notably, the investigators demonstrate that NMN’s beneficial effects rely on Sirt3 activation, highlighting a potential mechanism of action.
NMN Boosts Mitochondria and Reduces Senescence in Old Stem Cells
Dysfucnfucntional mitochondria hinder the production of ATP, our cell’s energy currency. Moreover, they exacerbate the production of reactive oxygen species (ROS), harmful compounds that induce oxidative stress. These consequences are known drivers of accelerated aging and have been shown to promote cellular senescence. With this in mind, the investigators examined whether treating old stem cells with NMN could improve mitochondrial function and limit senescent cell burden.
Prior to treatment, the old stem cells exhibited decreased ATP production and increased ROS levels, indicating poor mitochondrial function. Following treatment, the old stem cells had significantly higher ATP production and drastically lower ROS levels. Furthermore, NMN treatment lowered the number of senescent cells in old stem cells. Taken together, the initial findings demonstrate that NMN attenuates cellular senescence by restoring mitochondrial function.
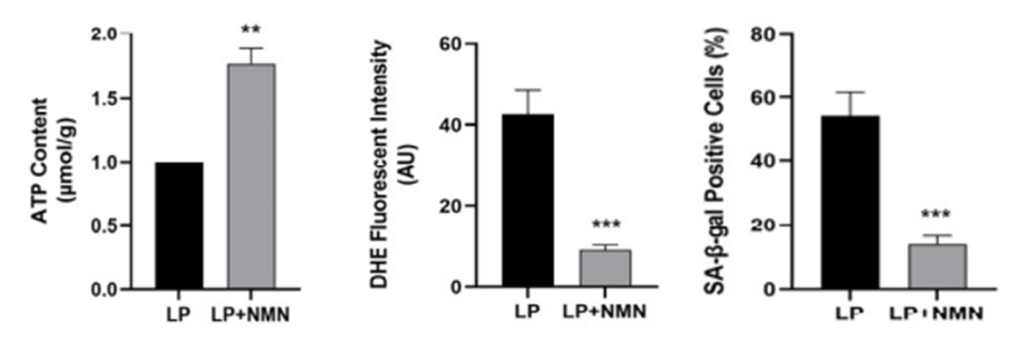
NMN’s Beneficial Effects Rely on Sirt3 Activation
Sirt3 is a critical mitochondrial protein that helps regulate oxidative stress and plays a key role in ATP production. Given Sirt3’s involvement in these critical processes, Wang and colleagues tested whether NMN altered Sirt3 activity in old stem cells. Accordingly, Sirt3 activity was significantly higher in old stem cells treated with NMN, highlighting a potential connection between Sirt3 activation and the observed mitochondrial benefits following NMN treatment.
To further elucidate whether Sirt3 activation governed NMN’s mitochondrial benefits, the investigators examined whether inhibiting the Sirt3 protein in old stem cells would reverse NMN’s effects on mitochondria following treatment. The results showed that blocking sirt3 abolished the effects of NMN, suggesting the effects of NMN are mediated by Sirt3.
Overall, the findings highlight a potential mechanism linking Sirt3 activation, healthy mitochondria, and decreased senescence in old stem cells.
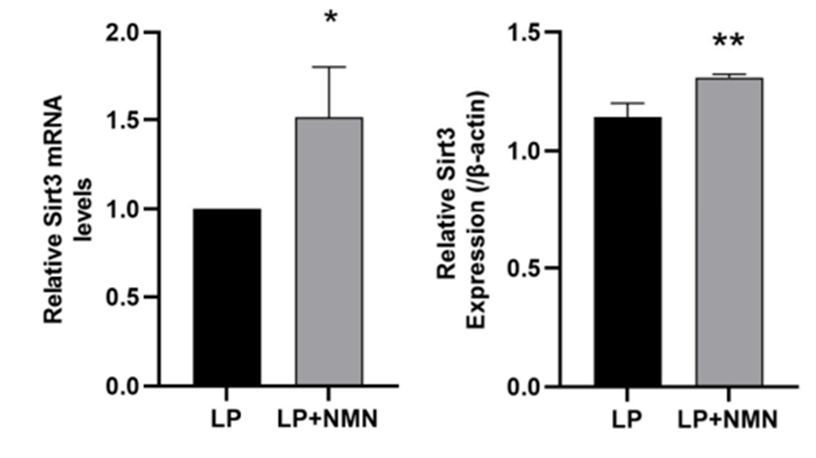
Sirtuin Activation and Longevity
Studies continue to demonstrate the importance of sirtuins in increasing longevity. Notably, sirtuins are critical to repairing and maintaining the integrity of our genetic blueprints (DNA), which drive the majority of age-related diseases when compromised. However, sirtuins require NAD+ for activation. Thus, NAD+ precursors like NMN are prime candidates to spark sirtuins health-boosting effects. In the present study, NMN’s ability to enhance mitochondrial function and decrease stem cell senescence through Sirt3 regulation demonstrates that NAD+ precursors could restore the therapeutic potential of stem cells and delay aging features by activating sirtuins.
Model: Early passage and late passage stem cells derived from health, 1-2 month-old male Wistar rats bone marrow.
Dosage: 100 µM NMN for 48 hours in culture.

