New Target for Non-Replicating Cells Improves Various Age-Associated Disorders
Elimination of growth-arrested cells by inhibiting a metabolic pathway improves age-related tissue dysfunction
Highlights
- Metabolism of the protein building block glutamine is essential for the survival of non-replicating, growth-arrested human cells termed senescent.
- Inhibition of glutamine metabolism induces the death of senescent cells and ameliorates aging-related organ dysfunction in mice.
Senescent cells — those that no longer replicate or grow — accumulate as we grow older and are linked to a variety of age-related medical conditions. The removal of senescent cells, what scientists call senolysis, has been proposed as beneficial for improving age-associated disorders and diseases like Alzheimer’s and cardiovascular disease. To target cells for senolysis, we need to have a detailed map on how cells become senescent.
Johmura and colleagues from The University of Tokyo published an article in Science that added a new route that cells use to achieve senescence, and, in doing so, identified a potential way to target it for senolysis. The Japanese researchers identified a key role for a type of metabolism based on glutamine — a protein building block — for the survival of human senescent cells. They found that targeting a key enzyme in this pathway called glutaminase 1 (GLS1) helped with aging-related organ dysfunction and obesity-related disorders in mouse models, suggesting the potential therapeutic value of this approach.
“Our results can contribute to the development of new anti-aging therapies that remove senescent cells by targeting these cells’ metabolic characteristics,” said Dr. Makoto Nakanishi, the senior author of the research article. “We hope that innovative anti-aging therapies and treatments for geriatric diseases will be developed that can remove senescent cells by treatment with GLS1 inhibitors.”
Glutamine metabolism is essential for cell senescence in human cells and mice.
The accumulation of senescent cells is linked to age-related disorders and diseases, which can be alleviated by compounds called senolytics — a class of molecules that can selectively induce death of senescent cells. But, not all cells that become senescent take the same path, and many of these senescence pathways have remained elusive.
To identify novel senolytic compounds, Johmura and colleagues performed a screen to identify enzymes that are essential for senescent cell survival. They applied a battery of molecules called short hairpin RNAs (shRNAs) that block the production of specific proteins to human senescent cells cultured in the lab and looked for which shRNAs induced cell death.
This screen for compounds that trigger senolysis identified a key role for the enzyme glutaminase 1 (GLS1), which is critical to glutamine metabolism — an important source of cell fuel. In concordance with these findings, the researchers found that the protein levels of GLS1, as well as other enzymes implicated in glutamine metabolism, were increased in human senescent cells. To make sure that their screen was no fluke, Johmura and colleagues confirmed the results they saw using shRNAs targeting GLS1 with complementary methods that blocked glutamine metabolism by depleting GLS1 levels as well as inhibiting its activity.
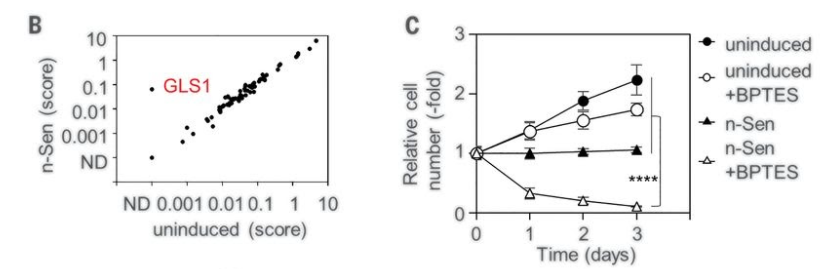
Johmura and colleagues then took their investigation from human cells cultured in dishes to rodents. They compared the levels of senescent cells and markers for glutaminase metabolism in different tissues between aged (80-weeks) and young (12-weeks) mice. The researchers found an increase in senescent cells in the skin, fat, and lungs of aged mice that also were positive for markers of glutamine metabolism. When they injected these aged mice with a GLS1 inhibitor, they saw a remarkable decrease in the levels of these senescent cells in the aged mice.
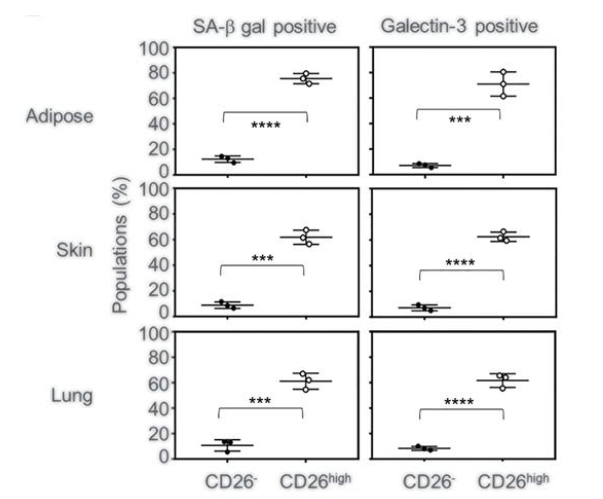
Senolysis by inhibition of glutamine metabolism ameliorates age-associated disorders
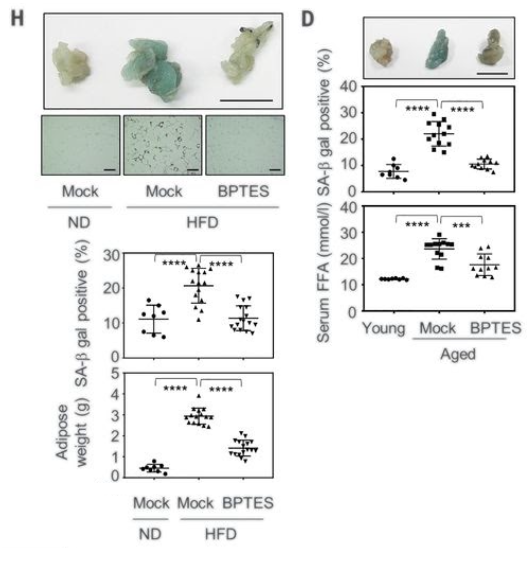
The researchers then examined the effects of inhibiting glutamine metabolism on organ dysfunction in aged mice. When they administered the GLS1 inhibitor, Johmura and colleagues saw ameliorated age-associated dysfunction of the kidney and lungs as well as fat tissue. The muscles of these aged mice treated with the GLS1 inhibitor also had improvements in muscle abilities.
Since senolytic drugs have been reported to alleviate obesity-induced disorders in addition to age-related disorders, the researchers looked at how inhibition of glutamine metabolism affected high-fat diet induced dysfunction. One month of injections with GLS1 inhibitors in obese mice (48-week-old mice on high-fat diets) had improved fat tissue as well as glucose tolerance and insulin sensitivity.
Johmura and colleagues also looked at the effects of glutamine metabolism inhibition in a couple other mouse models of disease like atherosclerosis and a type of liver disease called non-alcoholic steatohepatitis (NASH). In the atherosclerosis model, they saw improvements in the health of the aorta, which is typically littered with plaque and lesions in these mice, and they saw improved liver function in mice with NASH.
This study shows that, in addition to the clearance of senescent cells, glutamine inhibition provides protection from senescence improved age- and senescence-associated pathogenesis.
“Our results suggest that senescent cells rely on [glutamine metabolism], and its inhibition offers a promising strategy for inducing senolysis,” concluded the authors.

