Niacin Limits Alzheimer’s in Mice
Indiana University School of Medicine study shows that a form of vitamin B3 potentially paves the way toward treating this devastating neurodegenerative disease.
Highlights
- HCAR2 gene inactivation in Alzheimer’s mice exacerbates cognitive impairments and amyloid-β burden.
- HCAR2 activation using the FDA-approved drug NIASPAN® (niacin extended-release tablets) has protective and therapeutic effects in mice, modulating the microglial response to amyloid pathology.
- The results suggest that modulating microglia activity through HCAR2 might effectively prevent or treat Alzheimer’s.
If you open the cooler at any gas station and pull out an energy drink like Red Bull, Monster, or Rockstar, there’s a good chance that the beverage will be loaded with niacin, a soluble form of vitamin B3. Since we usually have an energy drink to heighten the activity of our brain, it may come as no surprise that one of the essential vitamins contained within could protect our brain from deterioration.
Indeed, Indiana University School of Medicine researchers found that niacin limits Alzheimer’s disease progression when used in a mouse model, a discovery that could potentially pave the way toward therapeutic approaches to the disease. The study, recently published in Science Translational Medicine, investigates how an FDA-approved version of niacin called NIASPAN® (niacin extended-release tablets), which modulates the response of microglia – specialized immune cells in the nervous system that remove damage and infection – to amyloid plaques in an Alzheimer’s disease animal model. This study shows that niacin is a promising therapeutic agent for Alzheimer’s disease with a high translational potential for clinical use.
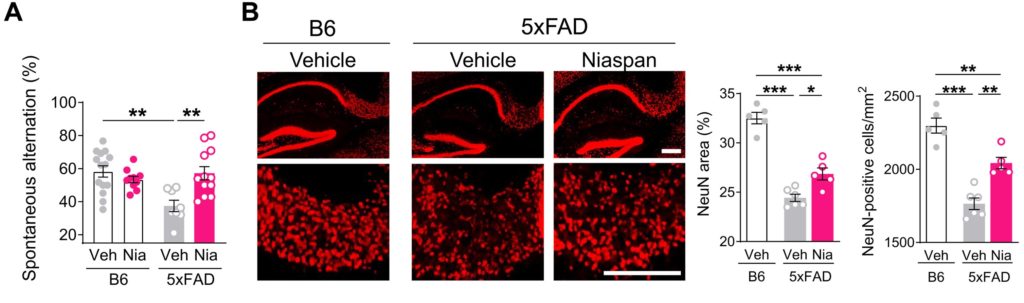
(Moutinho et al., 2022 | Science Translational Medicine) Niacin (NIASPAN®) rescued working memory deficits in Alzheimer’s mice. (A) NIASPAN® did not alter working memory in healthy, non-Alzheimer’s mice (B6), but the treatment of Alzheimer’s mice (5xFAD) with NIASPAN® rescued working memory deficits, demonstrating a robust therapeutic effect of niacin. (B) The behavioral improvement in 5xFAD mice treated with NIASPAN® was accompanied by reduced neuronal loss in the subiculum – a brain region key in mediating communication between brain regions for learning and memory (hippocampal) and higher-level processes (cortex) – analyzed by counting the number of mature neurons (NeuN).
“This study identifies a potential novel therapeutic target for Alzheimer’s disease, which can be modulated by FDA-approved drugs,” said Miguel Moutinho, Ph.D., a postdoctoral fellow at the Indiana University School of Medicine. “The translational potential of this strategy to clinical use is high.”
The Role of Microglia in Alzheimer’s
Alzheimer’s disease is the most common form of dementia for which there is no effective treatment. Mounting evidence suggests that the accumulation and aggregation of amyloid-β (Aβ) is a key initiating factor in a cascade of events that lead to Alzheimer’s disease. The Alzheimer’s brain is typified by a robust microglial immune response triggered by Aβ accumulation. Furthermore, genetic studies have linked many immune genes subserving this microglial response to the risk of Alzheimer’s disease. Although microglia have emerged as an important player in Alzheimer’s disease development and progression, the role of these cells in the disease is complex and still not fully understood.
Niacin Effectively Treats Alzheimer’s Mice
Since the increased dietary intake of niacin has been correlated with reduced Alzheimer’s risk, Moutinho and colleagues investigated how dietary intake of niacin reduced the risk of developing Alzheimer’s. Niacin is known to work by binding a receptor called HCAR2, which is expressed in the brain and has been shown to modulate microglial actions in several central nervous system disease models. Specifically, HCAR2 activation with niacin elicits several responses in microglial cells, including the secretion of inflammatory molecules and the monitoring and destruction of foreign substances. But not much has been known about whether HCAR2 modulation can affect microglial functions in Alzheimer’s.
Levels of the niacin receptor HCAR2 are increased in the brains of patients with Alzheimer’s disease and rodent models. The increase in HCAR2 expression is robustly induced by amyloid deposition that starts very early in Alzheimer’s progression, and to a lesser extent, by tau pathology that is present at later stages of Alzheimer’s progression. HCAR2 activation using the FDA-approved version of niacin called NIASPAN® had protected Alzheimer’s mice by modulating the microglial response to amyloid pathology. Moreover, NIASPAN® activation of HCAR2 in Alzheimer’s mice led to reduced amyloid and neuronal loss as well as the rescue of working memory deficits. The results suggest that NIASPAN® may be a promising therapeutic approach to Alzheimer’s specifically targeting the neuroimmune response.
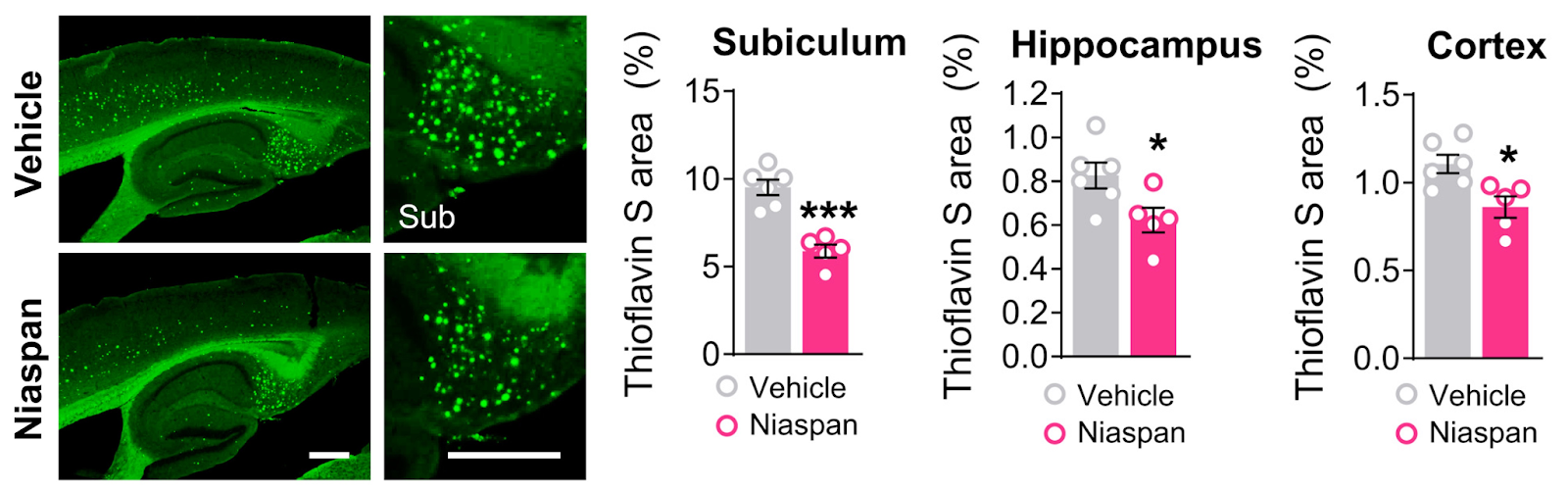
(Moutinho et al., 2022 | Science Translational Medicine) Niacin (NIASPAN®) stimulates microglia response and reduces amyloid pathology in Alzheimer’s mice. Imaging of thioflavin S–positive plaques revealed that treatment of NIASPAN® reduced amyloid plaque number and area, most prominently, not only in the subiculum but also in the hippocampus and cortex.
Moutinho and colleagues report that the HCAR2 is required for efficient microglia proliferation, engagement with amyloid deposits, and engulfment of amyloid-β, which are important microglial features reported to be beneficial in Alzheimer’s. Inactivating the HCAR2 gene in Alzheimer’s disease mice exacerbated cognitive impairments and amyloid-β burden. These data provide direct evidence that HCAR2 is required for an efficient and neuroprotective response of microglia to amyloid pathology.
Niacin Might Elicit Therapeutic Effects in Alzheimer’s
The present study reports a therapeutic strategy for Alzheimer’s tailored to potentiate microglia neuroprotective actions through the stimulation of HCAR2. The pharmacological activation of HCAR2 could be achieved by an FDA-approved formulation of niacin, NIASPAN®, with relatively minor adverse side effects, which might be repurposed for Alzheimer’s in the clinical setting. The strategy to pharmacologically stimulate HCAR2 in Alzheimer’s takes advantage of the receptors abundance in Alzheimer’s brains, inducing a robust microglia response and their beneficial actions.
Furthermore, according to the human equivalent doses based on body surface area, the dosage of NIASPAN® used to treat mice corresponds to a daily dosage of about 500 mg of NIASPAN® in humans, which is well below the daily maintenance dose used to treat dyslipidemia (1000 to 2000 mg). Moutinho and colleagues state, “Our findings suggest that a low and safe dose of NIASPAN® might be sufficient to elicit therapeutic effects in Alzheimer’s disease and are consistent with the existing epidemiological data showing that enhanced dietary niacin is associated with reduced risk for Alzheimer’s disease.”

