A Combination Treatment Has Promising Effects In ALS Patients
Clinical trial shows that nicotinamide riboside (NR) and pterostilbene treatment slowed the progression of ALS and even improved symptoms
Highlights
- This clinical trial tested the effects of EH301 — a combination of nicotinamide riboside (NR) and pterostilbene — in a small cohort of ALS patients.
- Several months of EH301 was shown to significantly slow the progression of ALS relative to placebo and even showed improvements in several key outcome measures.
Amyotrophic lateral sclerosis (ALS), or Lou Gherig’s disease, is a devastating neurodegenerative disease characterized by progressive loss of spinal and cortical motor neurons, leading to muscular deterioration, respiratory failure, and, ultimately, death. Although it has been nearly 150 years since the symptoms of ALS were first described, we have struggled to pinpoint a curative treatment for this debilitating and life-threatening disease.
De La Rubia and colleagues published results from a clinical trial (NCT03489200) in the journal Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration showing that, compared to placebo, ALS participants treated with a combination of nicotinamide riboside (NR) and pterostilbene demonstrated significant improvements in disease severity. These participants showed better pulmonary function, muscular strength, and skeletal muscle to fat weight ratio. This study provides evidence in support of the disease-modifying effects of this combination treatment of NR and pterostilbene for the treatment of ALS.
“Our preliminary results show its positive effect against ALS progression and provide support for a larger study to formally test treatment efficacy,” concluded the authors.
What are NR and pterostilbene used for?
There is substantial interest in proteins that depend on the vital molecule nicotinamide adenine dinucleotide (NAD+) called sirtuins as therapeutic targets in ALS. These longevity associated proteins regulate a myriad of cellular processes that are implicated in ALS, such as the regulation of energy metabolism, mitochondria function, inflammation, and DNA repair. Reduced levels of one particular sirtuin, sirtuin 1 (SIRT1), have been observed both in mouse models of ALS and postmortem tissues from ALS patients. So, techniques to increase sirtuin activity represent promising therapeutic approaches in the treatment of ALS.
How was the clinical study designed?
Along these lines, De La Rubia and colleagues evaluated the safety, effectiveness, and tolerability of a candidate drug that promotes sirtuin activity in people with ALS. This drug called EH301 consists of NR (1000 mg) and pterostilbene (200 mg). NR has been shown to increase circulating levels of NAD+ in published human clinical trials, and pterostilbene has been shown to directly activate SIRT1. So, in theory, NR and pterostilbene should work in tandem to boost NAD+ levels to drive a strong and steady activation of SIRT1.
The clinical trial began with 32 ALS patients, and 17 were treated with the NR and pterostilbene cocktail while the remaining 15 received placebo. Following baseline evaluation, study participants were evaluated following 2 and 4 months of treatment. The primary outcome measurement for the research team was a score of disease severity. Secondary to this, they wanted to get a good grasp of some other relevant measurements, including pulmonary function, muscular strength, body mass index (BMI), fat and skeletal weight, and electrical activity of skeletal muscles. After 4 months, several members from both groups exited the trial due to extenuating circumstances, which left 10 members in each group.
NR and pterostilbene improve ALS outcome measures
De La Rubia and colleagues observed a major improvement in the disease severity score in participants treated with NR and pterostilbene relative to placebo following 4 months of treatment. At this time-point, participants within the placebo group had deteriorated significantly relative to their baseline measurements, whereas the EH301 group showed no significant decline. When comparing the change from baseline — at the start of the trial before treatment — between EH301 and placebo, a significant improvement was observed in the EH301 group at the 2- and 4-month evaluation.
The research team observed a significant slowing or reversal associated with treatment with NR and pterostilbene in other outcome measurements. At both 2 and 4 months, participants in the EH301 group showed a significant improvement in muscular strength relative to placebo despite the baseline being lower in the EH301 group.
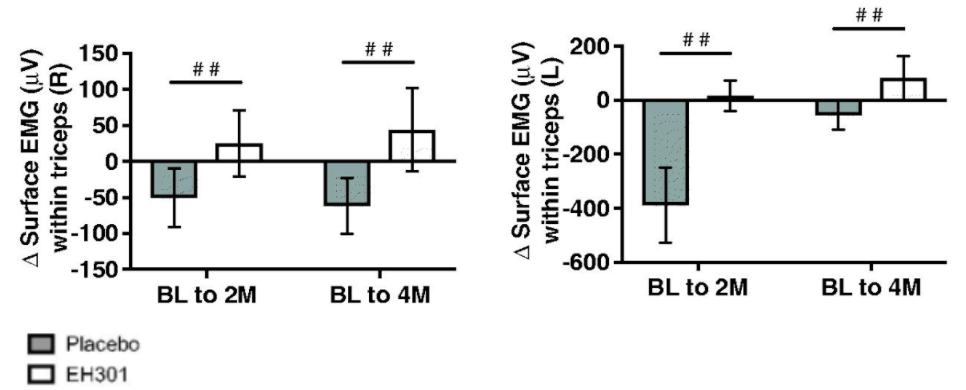
De La Rubia and colleagues observed improvements at both 2 and 4 months in pulmonary function and electrical activity of skeletal muscles. They also noted that treatment with NR and pterostilbene induced a significant decrease in fat and a significant increase in skeletal muscle weights, effects opposite to the placebo group at both 2- and 4- months.
Importantly, all participants enrolled in the trial exhibited a positive response in at least one of the outcome measurements. These observations support the findings from the main clinical outcomes, indicating that the participants with ALS within the EH301 group were gaining muscular strength.
Opting in for further treatment with NR and pterostilbene
After this study concluded in June 2017, all participants were given the option to continue treatment on an open-label extension study. All study participants elected to continue taking NR and pterostilbene. In February 2018, the research team completed a 1-year evaluation of participants initially randomized to EH301.
Relative to baseline, De La Rubia and colleagues did not observe any significant deterioration in the score for disease severity or muscle function. Also, 6 of the 8 muscle groups investigated using EMG did not show deterioration. They did, however, detect a reduction in forced vital capacity (FVC) — the amount of air that can be forcibly exhaled from your lungs after taking the deepest breath possible. This suggested that the participants experienced some decline in pulmonary function between baseline and 1 year. However, this reduction in FVC at 1-year is still less than the reduction in FVC observed in the placebo group at 4 months.
What’s next for ALS treatment?
This clinical study shows that treatment with NR and pterostilbene is safe and has promising effects for ALS patients. These preliminary results show the positive effect of NR and pterostilbene against ALS progression and provide support for a larger study to formally test treatment efficacy. All participants treated with NR and pterostilbene demonstrated improvement in at least one outcome measure, with the majority of participants showing improvement in at least two clinical measures.
But the small sample size of this study is insufficient to achieve definitive results, so more investigation is necessary. The findings from this pilot trial must be further validated in a larger clinical trial to explore the efficacy of NR and pterostilbene in a larger patient population.