NMN Makes Ears Resistant to Chemotherapy Poisoning in Mice
New research reveals that nicotinamide mononucleotide protects cells critical for hearing and balance from damage caused by the chemotherapeutic cisplatin.
Highlights
· Cisplatin causes ototoxicity — ear poisoning — and reductions in the cell essential molecule nicotinamide adenine dinucleotide (NAD+).
· The NAD+ precursor nicotinamide mononucleotide (NMN) protects cochlear cells from cisplatin-induced ototoxicity in mice, preserving hearing.
There are few, if any, silver bullet clinical treatments for cancer, as many medical interventions have side effects. For example, the chemotherapeutic cisplatin can cause hearing loss and ototoxicity — when a medical treatment causes hearing or balance problems. This ototoxic agent causes damage to the specialized cells in the snail shell-shaped structure in our ears (the cochlea) that harbor cells that are sensitive to sound (hair cells).
New research from Zhan and colleagues shows that the levels of a critical molecule to cell function and survival called nicotinamide adenine dinucleotide (NAD+) drop in hair cells treated with cisplatin, eventually leading to declines in hair cell number and function. However, when the researchers from Sun Yat-Sen University in Guangzhou, China, injected mice with NMN (100 mg/kg), a precursor of NAD+, the hair cells survived, and the mice retained much of their hearing ability.
“These results suggested that direct modulation of cellular NAD+ levels could be a promising therapeutic approach for protection of hearing from cisplatin-induced ototoxicity.”
Cisplatin Induces Cancer Patient Hearing Loss
Cisplatin and similar platinum-based drugs are prescribed for an estimated 10 to 20% of all cancer patients. The drugs cause permanent hearing loss in 40 to 80% of adult patients and at least half of the children who receive the drug. To reduce cisplatin-induced ototoxicity, many challenges remain, including a lack of understanding of how cisplatin causes ototoxicity, and the selection of an optimal treatment strategy has yet to be determined.
NAD+ level drops in the cisplatin-treated mouse cochlea
In this study, Zhan and colleagues dissected how cisplatin treatment affected the cochlea in mice. To do so, they extracted the cochlea from mice and moved them to laboratory dishes, where they added cisplatin and tracked how this affected hair cells.
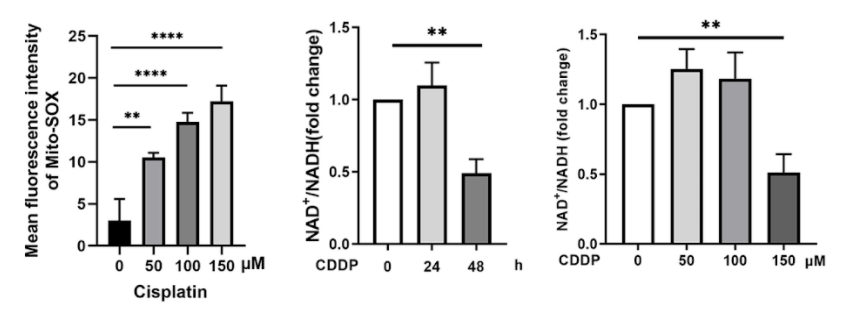
What the Sun Yat-Sen University researchers found was that cisplatin caused the death of hair cells in a time- and concentration-dependent manner; that is, the toxicity of cisplatin increased with longer and more concentrated exposures. Cisplatin also caused dysfunction of mitochondria, important cell structures that take charge of vital functions like energy production and protection against oxidative stress — where harmful, oxygen-containing molecules accumulate.
Zhan and colleagues also looked at how NAD+ levels were correlated to cisplatin ototoxicity because NAD+ has emerged as a potential therapeutic molecular target in various diseases. Although small concentrations and short lengths of cisplatin treatment led to slight increases in NAD+ levels, the highest concentrations and longest treatments of cisplatin led to major decreases in NAD+ levels.
NMN protects against cisplatin-induced hair cell loss and hearing loss in mice
To study the relationship between cisplatin-mediated hair cell death and NAD+ contents, Zhan and colleagues stimulated different NAD+ synthesis pathways. Several compounds were able to increase NAD+ contents in cochlear explants, including the NAD+ precursor NMN. In addition, supplementation of a different intermediate L-Tryptophan and a specific inhibitor (TES-1025) of an enzyme that limits NAD+ synthesis also increased NAD+ levels. However, direct NAD+ supplement to cochlear explants had little effect on intracellular NAD+ level.
In addition to reductions in hair cell number, cisplatin treatment caused noticeable defects in the hearing abilities of mice. But in mice administered cisplatin, NMN treatment led to smaller hearing threshold shifts, which are indicative of increased hearing sensitivity, compared with the group that didn’t receive NMN.
“Our data support that the modulation of biosynthesis of NAD+ protects against cisplatin-induced ototoxicity,” concluded Zhan and colleagues.
Interestingly, in a recent clinical study, supplementation with NMN partially improved the auditory capacity of elderly men. With a better understanding of how “ear poisoning” occurs and clinical testing of NMN in humans treated with chemotherapeutics, better treatment regimens can be designed to protect hair cells from damage and maintain hearing abilities in cancer patients or any person receiving an ototoxic treatment.

