NMN Limits Breast Cancer Growth and Spread, According to Chinese Study
Researchers find NMN impedes the growth and spread – metastasis – of a common, hard-to-treat form of breast cancer and improves survival odds with cancer in mice.
Highlights
- NMN inhibits the growth of a common breast cancer form – triple negative breast cancer (TNBC) – that evades typical treatments.
- NMN significantly improves tumor-bearing mouse survival following breast cancer injection.
- NMN reduces breast cancer metastasis to lung tissue by activating the pro-longevity protein Sirtuin 1, which repairs DNA and eliminates cell stress.
Breast cancer – a horrendous diagnosis – has surpassed lung cancer as the most common cancer form in the last few years. Even more troubling is that the triple negative form of breast cancer, accounting for ~15% of cases, evades typical treatments, leaving chemotherapy as one of the only options. This circumstance has created the need for safer and more effective ways to treat TNBC.
Published in Oncogene, Luo and colleagues from Tsinghua University in China show that NMN limits TNBC tumor growth in mice. Moreover, they show that NMN significantly improves survival chances following cancer onset and thwarts the metastasis of cancer. If these findings can translate to humans, they may provide hope for a new way to mitigate the growth and spread of TNBC.
NMN Slows Tumor Growth and Spreading While Increasing Survival Odds
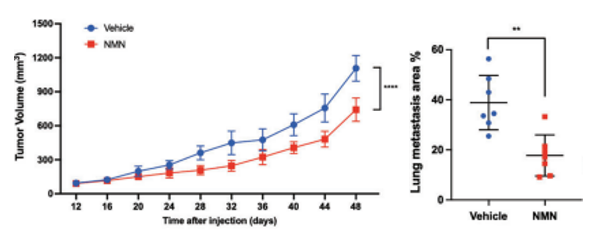
To test how NMN affects TNBC tumors, the researchers injected immunodeficient mice with human TNBC cells and measured tumor volumes while the mice were administered 500 mg/kg/day doses of NMN. At 48 days after tumor cell injections, NMN reduced tumor volume over 10% compared to tumor-bearing mice not given NMN. Moreover, NMN cut tumor growth and spread in lung tissue in half. These findings support that NMN significantly slows TNBC tumor growth and substantially limits its metastasis to other tissues.
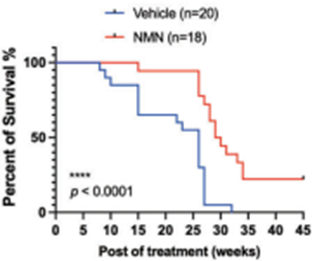
Luo and colleagues sought to find how NMN’s capacity to slow tumor growth and spread affects lifespan. They found that NMN extended the median remaining lifespan by ~15% after the cancer cell injection. These results suggest that by thwarting cancer proliferation, NMN improves lifespan following the onset of TNBC.
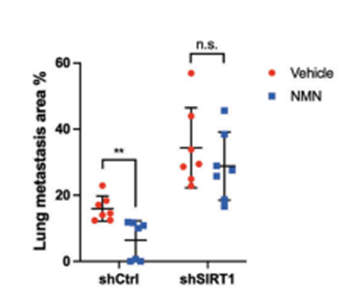
Increasing nicotinamide adenine dinucleotide (NAD+) levels with the NMN precursor activates the pro-longevity protein Sirtuin 1, which repairs DNA and eliminates harmful molecules in cells called reactive oxygen species. For this reason, the China-based research team proposed that NMN’s anti-cancer benefits stem from activating Sirtuin 1. Along those lines, they genetically eliminated Sirtuin 1 and found that without this protein, NMN didn’t significantly impede TNBC metastasis. However, by leaving Sirtuin 1 in place, NMN substantially hinders TNBC metastasis. This means that NMN slows cancer and metastasis likely by driving Sirtuin 1 function.
“We discovered that NAD+ supplementation suppressed TNBC progression,” said Luo and colleagues.
NMN’s Anti-cancer Properties May Depend on Dosage
Interestingly, Luo and colleagues found that although NMN reduces tumor growth, it doesn’t necessarily halt tumor cell proliferation. This raises the possibility that NMN increases the anti-cancer immune cell response, leading to suppressed tumor growth. What’s more, in another study that used a lower dose of 250 mg/kg/day, NMN had no impact on tumor growth. This means that higher doses like the one used in this study may be required for NMN to exert its full anti-cancer benefits.
Because abrasive chemotherapy is typically currently used to treat TNBC, the possibility that NMN may slow TNBC tumor growth and spread presents a welcome addition to the anti-cancer compound repertoire. Future clinical studies will help determine whether NMN can be used against human TNBC.
“Our findings provided evidence that targeting NAD+ metabolism could be an effective therapeutic intervention against TNBC, hoping to translate the results to humans,” said Luo and colleagues.
Model: NCG mice that were six to eight weeks old
Dosage: 500 mg/kg/day for 45 weeks

