NMN-Loaded Vesicles Shown to Delay Skin Aging in New Study
New research reveals that small vesicles derived from human umbilical cord cells, when infused with NMN (nicotinamide mononucleotide), delay skin aging in a mouse model.
Highlights
- Treating skin with NMN-loaded vesicles counteracts wrinkling and thinning of skin tissue in a mouse model of skin aging.
- Treatment with the NMN-loaded vesicles also reduces protein markers of aging and signs of DNA damage in the model of skin aging.
Published in Stem Cell Research and Therapy, Tan and colleagues from Jiangsu University in China show that small vesicles from human umbilical cord cells (sEVs) loaded with NMN counteract skin wrinkling and thinning, both signs of aging, in a mouse model of skin aging. Additionally, the NMN-loaded sEVs reduced aging-related protein markers and prevented DNA damage in the aging skin model. These findings suggest that infusing sEVs with NMN may serve as a way to enhance the efficacy of NMN in counteracting signs of aging.
As we age, all of our body’s organs undergo functional deterioration, with skin aging being particularly apparent due to the skin’s external location on the body. As the body’s principal superficial organ, the skin maintains constant interaction with the surrounding environment while acting as a barrier against dehydration, ultraviolet radiation, and pathogens. Moreover, both internal and external factors influence skin aging, facilitating deterioration in the skin’s structural integrity and reduced overall function.
With aging, dysfunctional cells that can emit inflammatory molecules, called senescent cells, accumulate in skin tissue. As such, senescent skin cells can transmit inflammatory molecules to other tissues and organs, thereby contributing to senescence throughout the body. For example, research has shown that the presence of senescent cells in the skin results in senescent cell buildup in tissues outside the skin, as well as increased frailty, impaired skeletal muscle function, and reduced cognitive function. Altogether, with skin aging’s potential to drive aging throughout the body via senescent cells, identifying ways to slow skin aging could serve as a key to generally counteract whole-body aging.
Along those lines, stem cell therapies have received attention in tissue regenerative research in recent years. In that regard, stem cell vesicles from umbilical cords have shown promise in protecting the skin against ultraviolet radiation-induced damage in a rat model. However, the capabilities of these vesicles to delay skin aging have remained limited.
In a similar vein, the NAD+ (nicotinamide adenine dinucleotide) precursor, NMN, has been shown to improve various age-related conditions in preclinical models. Some evidence also suggests that NMN protects against skin aging. However, NMN is believed to be unstable in circulation, which may hinder its benefits once orally ingested or injected. Thus, finding a way to stabilize NMN with sEVs could help unravel whether this combination helps delay skin aging better than either strategy alone.
To harness the potential of sEVs and couple them with NMN, Tan and colleagues loaded sEVs with NMN. In doing so, the China-based researchers hoped to capitalize on the prospect that the vesicles and NMN could delay skin aging, all the while potentially stabilizing NMN in circulation to enhance its effects.
“Our results provide theoretical justification for the utilization of [NMN-loaded sEVs] in anti-skin aging,” said Tan and colleagues in their publication.
NMN-Loaded Umbilical Cord Vesicles Delay Skin Aging
To examine the effects of NMN-loaded sEVs on skin aging, Tan and colleagues first generated a mouse model of skin aging. To do so, the researchers applied a simple sugar, D-galactose, to the shaved backs of mice. D-galactose induces skin aging primarily by increasing oxidative stress (arising from elevated levels of harmful, reactive molecules in cells) as well as the formation of advanced glycation end-products (molecules tied to aging that form when sugars react with proteins or fats).
Then, to deliver NMN-loaded sEVs, sEVs on their own, or NMN on its own to the skin, Tan and colleagues utilized nano-microneedles. Nano-microneedles use tiny, cone-shaped structures to create microscopic channels on the skin’s surface and infuse cargo. In this case, the researchers used nano-microneedles to infuse NMN-loaded sEVs, sEVs alone, or NMN deeper than topical application methods, such as creams.
With nano-microneedles as a delivery method, Tan and colleagues then treated skin aging model mice with NMN-loaded sEVs, NMN, or sEVs to gauge each treatment’s effects on preventing skin aging. As expected, the skin aging model mice that received no treatment exhibited fine wrinkles on their back skin. In contrast, groups that received either sEV or NMN treatment showed attenuated wrinkle folds on the shaved backs of the skin aging model mice. Intriguingly, the mice treated with NMN-loaded sEVs showed hardly any wrinkles, making their skin almost indistinguishable from typical mice without D-galactose-induced skin aging. These findings suggest that NMN-loaded sEVs delay skin aging to a better degree than sEVs alone or NMN on its own.
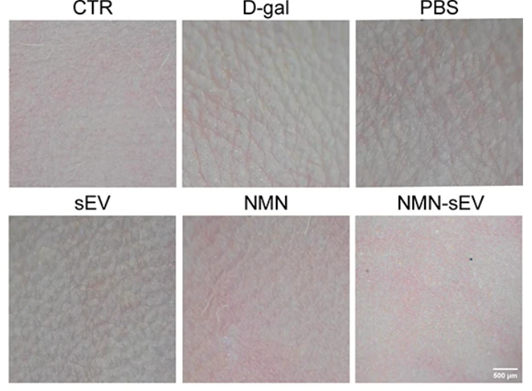
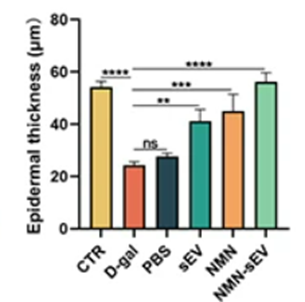
Because thinning of the skin is associated with aging, Tan and colleagues sought to measure how NMN-loaded sEVs, sEVs alone, and NMN alone affect skin thickness in the mouse model of skin aging. As expected, the researchers found that mice that underwent application of D-galactose to their shaved backs to induce skin aging exhibited significant skin thinning. Moreover, the sEV-treated, NMN-treated, and NMN-loaded sEV-treated groups showed reduced skin thinning, with the most significant improvement seen in the NMN-loaded sEV-treated group. These results suggest that NMN loaded into sEVs delays skin aging better than sEVs or NMN on their own.
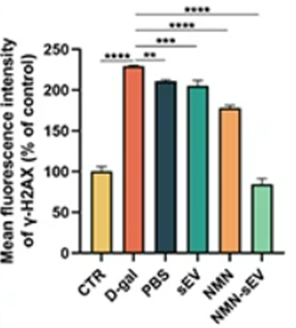
To confirm that NMN-loaded sEVs alleviate aging at the cellular level in skin, Tan and colleagues measured cellular markers of cellular senescence—a dysfunctional cellular state linked to aging. The researchers also examined how NMN-loaded sEVs affect a cellular marker of DNA damage since DNA damage is tied to aging. According to assessments of the cellular markers of senescence and DNA damage, D-galactose-induced skin aging indeed increased markers of senescence and DNA damage in skin cells. However, NMN-loaded sEV treatment, sEV treatment, and NMN treatment alleviated senescence and DNA damage, with NMN-loaded sEVs conferring the greatest effect. These findings suggest that, at the cellular level, NMN-loaded sEVs, incorporating both NMN and sEV strategies, additively alleviate signs of aging, like cellular senescence and DNA damage, better than sEVs or NMN on their own.
Orally Ingesting NMN-Loaded Umbilical Cord Vesicles May Serve as a Way to Delay Whole-Body Aging Processes
As the study’s findings suggest, NMN loaded into sEVs additively enhances the effects of either aging intervention strategy, sEVs or NMN, on its own in a skin aging mouse model. Since the researchers treated mouse skin with D-galactose to recapitulate skin aging, which is not the same as naturally aged skin, future research should explore whether NMN-loaded sEVs delay skin aging in the same way in the skin of aged mice. Tan and colleagues likely used the D-galactose skin aging mouse model since laboratory mice typically live for about two to three years. Hence, using the D-galactose mouse model of skin aging would take less time, requiring less laboratory work and financial resources.
As far as human application goes, clinical trials are still necessary to find whether NMN-loaded sEVs confer similar effects against skin aging in humans. Moreover, since loading NMN into sEVs may help stabilize NMN, clinical trials could also examine whether NMN-loaded sEVs work systemically to delay aging in organs outside of the skin.
Along those lines, research is ongoing to find whether humans can orally ingest sEVs to reap their potential therapeutic benefits. If such research finds that humans can indeed orally ingest sEVs, loading sEVs with NMN could serve as a new way to additively enhance sEV therapy and NMN supplementation to slow whole-body aging processes.
Model: Six-week-old ICR female mice with D-galactose applied to shaved backs twice daily for six weeks
Dosage: Once a week for six weeks, human umbilical cord sEVs (5×109 particles per mouse per week), NMN (2.2 mg per mouse per week), or NMN-loaded sEVs (5×109 particles per mouse per week) were applied to shaved backs via nano-microneedles

