NMN May Restore Bone Loss From Aluminum Toxicity
Scientists from China provide evidence: nicotinamide mononucleotide (NMN) treatment restores bone loss from aluminum consumption in rats.
Aluminum, an environmental contaminant, can have toxic effects on the brain, bones, liver, and kidneys.4,8,11 Medicines, cosmetic products, and food additives can contain aluminum.8,10 Acid rain has also mobilized aluminum from soil, making aluminum exposure through water and food products more prevalent.4 Approximately 50-70% of aluminum entering the body accumulates in the bone, a primary target for aluminum toxicity.7,8 Exposure of bone to aluminum results in bone impairment and disruption of bone formation, causing bone diseases such as osteoporosis, osteodystrophy, and osteomalacia.1,8
The molecular mechanism of aluminum-induced bone toxicity remains unclear.8 Previous studies indicate oxidative stress and inflammation contribute to bone toxicity and bone disease progression.3,8,9 Inhibiting oxidative stress and inflammation could prevent bone loss associated with bone toxicity.2,8,14
Studies indicate the precursor of nicotinamide adenine dinucleotide (NAD+) biosynthesis, nicotinamide mononucleotide (NMN), provides an effective therapy for improving intracellular NAD+ levels.8,13,15 Previous studies demonstrate decreased NAD+ levels associate with osteoblastogenesis, declined generation of bone cells. The present study investigates whether NMN supplementation of rats has protective benefits in aluminum-induced bone damage. The study also seeks evidence for the cellular pathways involved.
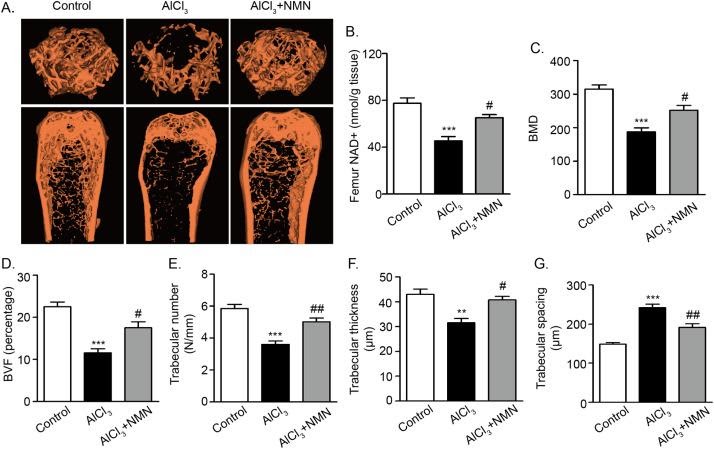
Image from Liang et al. (2019). In the image above, the top row has pictures looking at the top (epiphysis) of the femur bone, while the bottom row has pictures of the shaft (diaphysis) of the bone, taken with micro-CT imaging.
Results from experimentation indicate NMN treatment counteracts aluminum-induced bone impairment. The experiments demonstrate NMN supplementation restores reduced NAD+ levels in cells. Rats undergoing aluminum exposure treated with NMN have restored bone integrity.
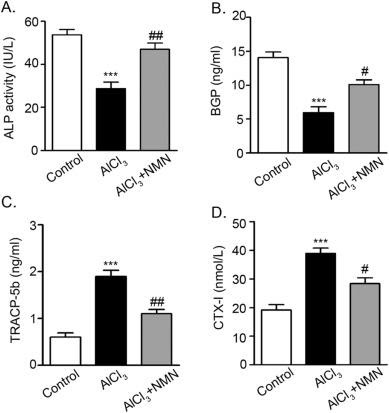
Image from Liang et al. (2019). The images above represent data collected in these experiments. They indicate ALP activity ecreases with exposure to aluminum.
Experimental results also indicate NMN treatment effectively alleviates aluminum-induced bone loss. ALP, an enzyme present at higher levels when bone grows, has increased levels after aluminum exposure with NMN treatment. NMN supplementation also restores BGP levels, an indicator for new bone formation, following aluminum exposure Researchers also tracked the level of TRACP-5b and CTX-1, which are markers that indicate bone breakdown in high levels. With NMN administration following aluminum exposure, both levels decrease.
Results from further experimentation in the study reveal aluminum-treated bone cells have higher levels of oxidative stress compared to cells without aluminum treatment. NMN treatment reduces the oxidative stress as measured with reactive oxygen species levels. Results also demonstrate NMN treatment inhibits cells involved in inflammatory responses in bone cells exposed to aluminum.
The results of the study demonstrate NMN can counteract adverse effects on bone of aluminum exposure. Humans may receive exposure to the inorganic contaminant, aluminum, through drinking water and food.5,8 Previous studies indicate oxidative stress, inflammation, bone impairment, and increased risk of osteoporosis result from aluminum exposure.8,12
The effects of NMN administration on bone impairment from aluminum exposure remains poorly understood based on previous studies. The results point to NMN treatment improving bone mineral density and structural properties receiving injury from aluminum exposure. NMN treatment also reduces oxidative stress from aluminum exposure, according to data from the study. The NMN treatment reduces expression of inflammation through inhibition of inflammasome activation. “NAD+ depletion might underlie [aluminum]-induced toxicity, whereas NMN may protect against [aluminum]-induced bone impairment via inhibiting the TXNIP-NLRP3 inflammasome pathway,” according to the authors of the study. The findings of the study provide new insight for using NMN to counteract aluminum toxicity.

