Study Zeroes In On NAD+ Metabolism and α-Ketoglutarate to Combat Obesity
Japanese researchers uncover a new role for NAD+ and α-Ketoglutarate in the development of fat
Obesity is a global epidemic that causes numerous metabolic diseases, including type 2 diabetes and cardiovascular disease. The excess accumulation of fat tissue that is characteristic of the disorder results from the increase in both the size and the development of mature fat cells called adipocytes. Discovering compounds able to regulate the size, number, and function of adipocytes and understanding how they act could greatly contribute to obesity prevention and treatment.
Okabe and colleagues from the University of Toyama in Japan published an article in the journal Frontiers in Cell and Developmental Biology showing that enhanced synthesis of nicotinamide adenine dinucleotide (NAD+), a molecule at the core of metabolism, is critical for the development of mature fat cells from precursors. “Our study uncovers a new role for NAD+ biosynthesis in adipogenesis and proposes a novel therapeutic approach to combat obesity,” said the researchers in the article.

What’s the Role of NAD+ in the Accumulation of Fat?
Adipogenesis—the maturation of adipocytes—is a finely controlled process. These cells are derived from precursors called preadipocytes, a process that is regulated by an elaborate network of gene modulating proteins called transcription factors that coordinate the expression of hundreds of proteins. When preadipocyte differentiation is disrupted, adipocytes swell and ectopic lipid accumulation occurs in inflammation and metabolic dysfunction.
It is also known that metabolic changes accompany adipogenesis. Although several studies have been performed to determine the metabolic changes that occur during the differentiation of preadipocytes, few have focused on the role of NAD+ metabolism.
Upregulation of NAD+ Synthesis Is Required for Preadipocyte Differentiation
In this study, Okabe and colleagues sought to elucidate the metabolic changes that occur during preadipocyte differentiation. They found that levels of NAD+ and nicotinamide mononucleotide (NMN), an NAD+ precursor, were significantly increased during the differentiation of preadipocytes. This was accompanied by increases in levels of the enzymes that synthesize both NAD+ and NMN. These data suggest that NAD+ synthesis is upregulated during preadipocyte differentiation.
Next, the researchers wanted to know if the increase in NAD+ level is required for preadipocyte differentiation. To address this, they blocked NAD+ production by inhibiting the enzyme that synthesizes NMN during the differentiation of preadipocytes. When they suppressed the rise of both NMN and NAD+ levels following the induction of differentiation, the differentiation of preadipocytes was considerably decreased. Moreover, NMN supplemented preadipocytes that had been induced to differentiate completely restored the NAD+ level and the differentiation of preadipocytes cells. These data demonstrate that increased NAD+ production is required for preadipocyte differentiation.
The Gene Program for Fat Cell Maturation is Regulated by NAD+ Synthesis
Okabe and colleagues next examined whether the adipogenic program was influenced by NAD+ levels. When NAD+ levels were reduced, the master regulator of adipogenesis PPARγ and target adipogenic genes were significantly suppressed. These results indicated that NAD+ must be upregulated to induce PPARγ and that NAD+ metabolism is involved in the induction of PPARγ during adipogenesis.
α-Ketoglutarate Regulates PPARγ Activation in Preadipocyte Differentiation
In order for the gene to be activated, the PPARγ DNA sequence must be demethylated, the removal of molecular tags called methyl groups that are bound to DNA. The researchers found that when demethylation is inhibited, the levels of PPARγ activation and preadipocyte differentiation are suppressed.
Also, the metabolite α-ketoglutarate is a known cofactor for demethylating enzymes. Interestingly, the levels of α-ketoglutarate significantly increased during preadipocyte differentiation, which correlated with increases in NAD+ levels. However, the reduction in NAD+ levels abolished the rise of α-ketoglutarate levels after differentiation was induced.
The researchers then wanted to see if there was a connection between NAD+, α-ketoglutarate, and adipogenesis. To do so, they supplemented preadipocytes with α-ketoglutarate. This rescued PPARγ levels and the differentiation of preadipocyte cells, all of which were suppressed by inhibiting NAD+ synthesis. Together, these data demonstrate that α-ketoglutarate levels, boosted by NAD+, are necessary for the demethylation of the PPARγ gene and lead to preadipocyte differentiation.
α-Ketoglutarate Prevents Diet-Induced Obesity by Promoting Appropriate Adipogenesis
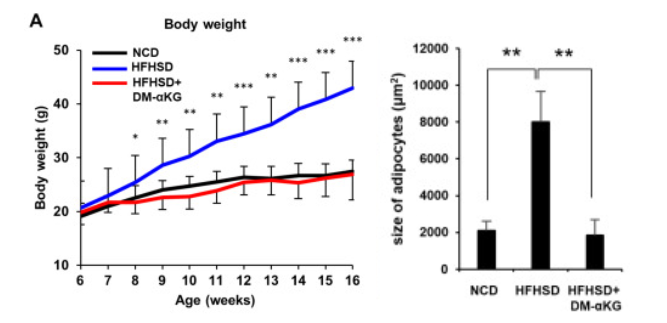
Finally, Okabe and colleagues studied how NAD+ and α-ketoglutarate influence obesity. Mice fed a high-fat high-sucrose diet that induces obesity were orally administered α-ketoglutarate in drinking water. Surprisingly, α-ketoglutarate administration completely suppressed the diet-induced obesity, resembling the mice fed a normal diet.
The researchers also examined the physical characteristics of fat tissues in these mice. The obesity-inducing diet caused the adipocytes to swell, while the size of adipocytes in the α-ketoglutarate administered group was significantly smaller. Additionally, the levels of genes in the adipogenic program were impaired in the mice fed obesity-inducing diets, which were rescued by α-ketoglutarate administration.
Can NMN or α-Ketoglutarate Prevent Obesity in Humans?
In conclusion, this study revealed a novel insight regarding how adipocytes mature involving NAD+-dependent metabolic reprogramming. “Our results indicate that the NAD+-α-ketoglutarate axis is a potential therapeutic target for obesity-related metabolic disorders,” said the investigators in the study. Further studies are required to explore whether these effects are reproducible in humans.