NMN Prevents Chemotherapy-Induced Cognition Impairments in Mice
Increasing NAD+ levels could represent a promising and safe therapeutic strategy for chemotherapy medicine-related neurotoxicity
Highlights
· Nicotinamide mononucleotide (NMN) prevents chemotherapy-induced abnormalities in brain cells and cognitive function.
· NMN’s protective effects on the brain and cognition did not affect tumor growth and anti-tumor efficacy of chemotherapy.
Cancer itself is a scary thing, and going through treatment isn’t exactly a walk in the park. Chemotherapy is taxing on the body and leads to all sorts of toxic effects like cognitive impairment, which has emerged as a significant medical problem. However, meaningful treatments are not currently available due to our poor understanding of how chemotherapy affects the brain to cause cognitive impairment.
In an article published in Cancer Research, Yoo and colleagues from the Mayo Clinic and the University of Pennsylvania show that chemotherapeutics suppress levels of the vital bioenergetic molecule nicotinamide adenine dinucleotide (NAD+) levels in adult mouse brains and human brain cells. But increasing NAD+ levels through nicotinamide mononucleotide (NMN) administration prevented abnormalities induced by the chemotherapeutic cisplatin in the brain at the levels of individual cells and tissue structure to cognitive function. Importantly, the beneficial effects of NMN did not interfere with the anti-tumor efficacy of cisplatin.
Yoo and colleagues propose, “Increasing NAD+ through nicotinamide mononucleotide supplementation offers a potential therapeutic strategy to safely prevent cisplatin-induced cognitive impairments, thus providing hope for improved quality of life in cancer survivors.”
The intersection of NAD+, chemotherapy, and aging
Proper brain function is highly dependent on controlled energy metabolism. NAD+ is an important metabolic byproduct involved in a plethora of processes linked to cell health, survival, and longevity. Unfortunately, levels of NAD+ and the enzyme that generates the NAD+ precursor NMN drop with age, which is a contributing factor in age-related diseases.
But, all is not lost, as it seems like these age-related impairments can be attenuated by increasing NAD+ through NAD+ precursors, such as NMN or nicotinamide riboside. Both precursor compounds have been reported as promising therapeutic compounds to delay aging, extend lifespan, and improve cognition.
Notably, chemotherapy detrimentally alters brain function similar to advanced aging. The two facilitate reduced brain cell growth, branching, and replication as well as increased neuroinflammation and memory dysfunction. Notably, chemotherapy accelerates biological aging and possibly cognitive decline, which has been reported in many breast cancer patients.
NMN prevents cisplatin-induced cognitive impairments in adult female mice
To test how exactly NAD+, chemotherapeutic, and brain health were interacting, Yoo and colleagues pretreated female adult mice with NMN by injecting a daily dose of 250 mg/kg followed by cisplatin treatment four hours later, which they repeated four times. They next used several tests to evaluate cognition, particularly memory, in rodent models of brain disorders, especially hippocampal impairments.
They first used the Novel Object Recognition (NOR) task, which is based on the rodent tendency to spend more time exploring a novel object than a familiar one. After exploring two familiar objects for a couple of days, the researchers then replaced one of the familiar objects with a novel object and measured how long they were willing to explore the new ones. Cisplatin-treated mice spent less time exploring the novel object compared to untreated mice, indicating that cisplatin impairs memory function. But when NMN was given to untreated and cisplatin-treated mice, both groups significantly increased exploration of the novel object, bolstering the evidence supporting that NMN enhances cognition.
To strengthen their results and avoid reliance on a single memory test, Yoo and colleagues then performed the Morris Water Maze, a more nuanced spatial memory test. In this test, rodents are scored for their ability to remember where a platform is hidden in murky water. Cisplatin-treated mice took longer to find the hidden platform and spent less time in the general vicinity of the platform, suggesting that cisplatin-treated mice displayed learning and spatial memory impairments. However, NMN administration before cisplatin-treatment significantly reduced the time to find the hidden platform and increased the time spent in the vicinity of the platform, further adding credulity to NMN’s effects on cognition.
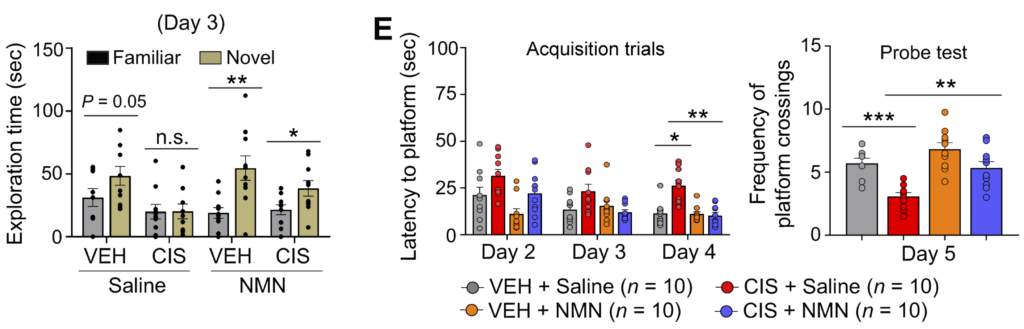
These tests showed a neuroprotective role for NMN in cisplatin-induced recognition and spatial memory dysfunction in female mice. Also, Yoo and colleagues observed that NAD+ is significantly reduced by cisplatin in the hippocampus and cerebellum, brain regions critical for memory and movement, respectively, which could be reversed by NMN treatment. This suggests that the observed neuroprotective effects of NMN on memory function are correlated with increased levels of NAD+ in the hippocampus and cerebellum of cisplatin-treated mice.
NMN does not impact the anti-tumor activity of cisplatin
Notably, NAD+ metabolism is known to play a role in cancer development. However, Yoo and colleagues did not find any detrimental impact of NMN on tumor growth and cisplatin’s antitumor activity, ensuring that NMN is a safe and promising compound for impaired cognition induced by chemotherapy.
“Taken together, our findings suggest that aberrant … NAD+ metabolic pathways may be a key contributor in cisplatin-induced neurogenic impairments, thus causally leading to memory dysfunction,” concluded Yoo and colleagues. Therefore, increasing NAD+ levels could represent a promising and safe therapeutic strategy for cisplatin-related neurotoxicity.”
Can NMN protect against cognitive impairment in chemotherapy patients?
Since other chemotherapies are associated with cognitive dysfunction, it is of importance to determine whether the neurotoxic effects of these drugs are mediated through dysregulated NAD+ metabolism. If this is the case, NMN could be a general therapeutic option to abate the neurotoxicity associated with other chemotherapies.
Nonetheless, NAD+ metabolism is actively being developed as a pharmacotherapy against aging and age-related diseases, and NMN is currently undergoing clinical trials to improve age-related metabolic dysfunction.
“Our … strategies to prevent NAD+ loss using NMN supplementation during cisplatin-chemotherapy is a promising therapeutic strategy that is rapidly and safely applicable to prevent cisplatin-induced neurotoxicity and improve quality of life for cancer patients.”
These studies will have to be recapitulated in humans and show that NMN is neuroprotective against chemotherapy-induced cognitive impairment in patients with cancer.

