NMN Reduces Heart Attack Damage by Activating Anti-Inflammatory Cells, New Study Shows
NMN (nicotinamide mononucleotide) triggers immune cells to reduce the cardiac damage caused by a heart attack in mice via a protein found in heart disease patients.
Highlights
- NMN induces the secretion of a protective protein called U2AF1 from immune cells that prevents heart damage.
- U2AF1 supports the survival of mice after a heart attack.
- Concentrations of U2AF1 are lower in heart attack patients.
Myocardial infarction (MI), or heart attack, occurs when a part of the heart dies due to a lack of blood supply, most commonly caused by a blood clot. The consequences can be severe, ranging from weakened heart function to death. Now, researchers have discovered how NMN contributes to reducing MI-induced cardiac tissue death.
NMN Triggers Anti-Inflammatory Immune Cells
Immune cells called macrophages are crucial players in the body’s healing process. After a heart attack, they rush to the site of injury, clear away dead tissue, and initiate recovery. However, while some macrophages take on a pro-inflammatory state, others enter an anti-inflammatory, reparative state. In this reparative state, macrophages secrete various factors that promote tissue regeneration and the formation of new blood vessels.
Researchers found that if macrophages were treated with NMN, they entered the anti-inflammatory, reparative state. This was shown by elevations in genetic markers for an anti-inflammatory molecule called Il10 and a reparative molecule called Vegf, which promotes the formation of new blood vessels. These findings suggest that the reparative function of macrophages can be significantly enhanced by NMN.
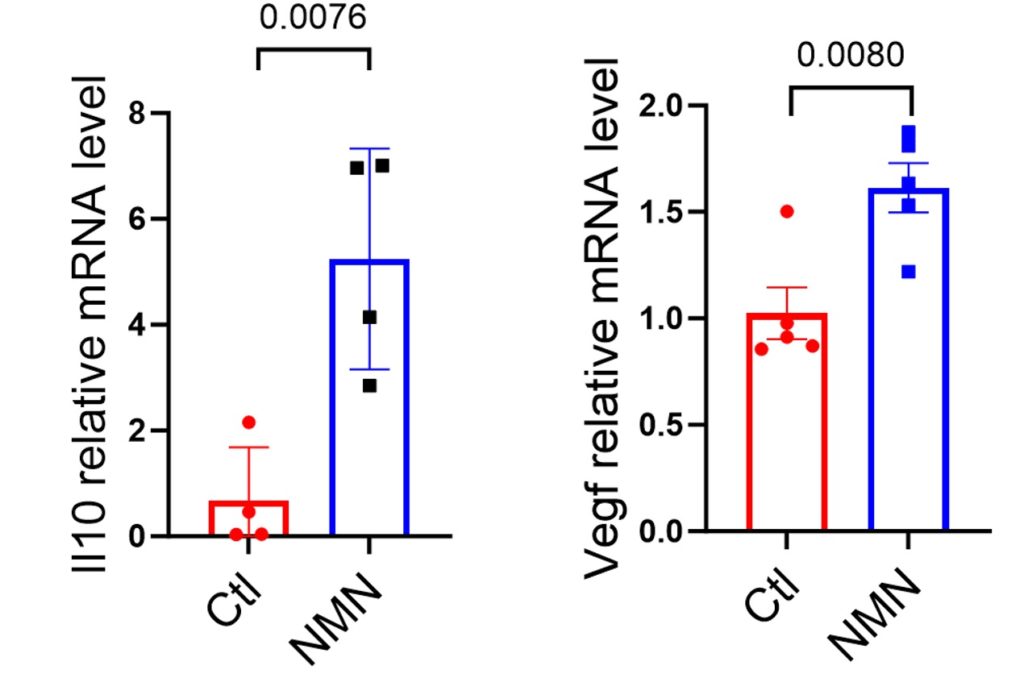
NMN Prevents MI-Induced Heart Tissue Damage
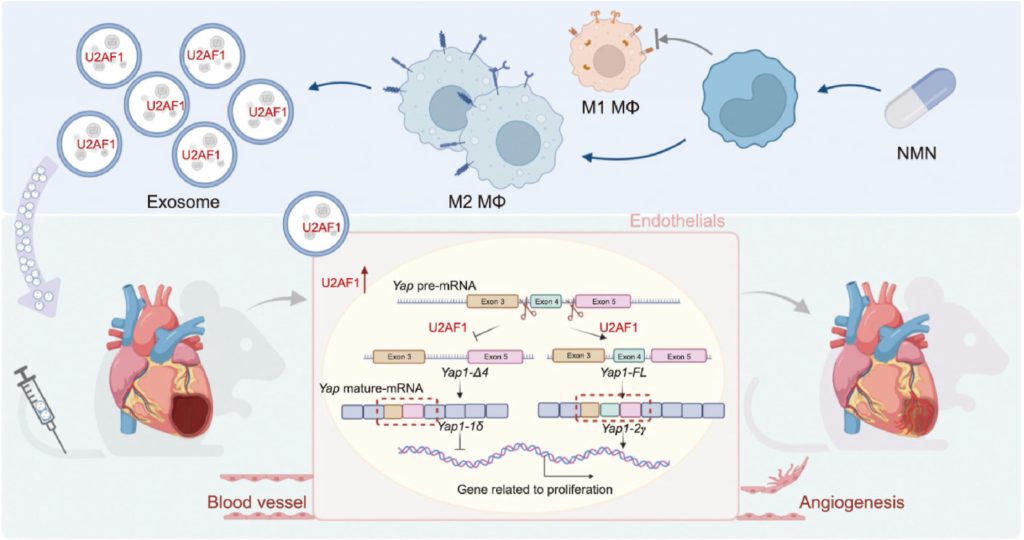
Beyond its direct influence on macrophages, NMN’s impact extends to cellular packages called exosomes. These nano-sized vesicles are essentially cellular messengers, carrying a cargo of proteins, lipids, and genetic material from one cell to another. They are a crucial part of body-wide communication and play a significant role in tissue repair.
The researchers found that NMN increases the secretion of exosomes from macrophages. Moreover, injecting these exosomes into a mouse model for MI led to improvements in heart function and repair. Interestingly, the exosomes improved cardiac function and reduced damage more than treatment with 5 to 500 mg/kg of NMN.
These findings confirm that NMN protects against MI, also revealing that exosomes from NMN-treated macrophages provide a potentially more potent protective effect. This suggests that NMN does not just improve the function of macrophages themselves, but also enhances the therapeutic potential of the exosomes they release. This exosome-based approach, boosted by NMN, could represent a promising frontier in regenerative medicine.

NMN Elevates a Protein that Supports Heart Repair and Survival
By exploring how exosomes from NMN-treated macrophages could support heart repair, the researchers identified a protein called U2AF1. They found that U2AF1 was elevated in exosomes from NMN-treated macrophages, suggesting its cardioprotective role. Confirming this role, the researchers showed that genetically increasing U2AF1 levels in the MI mouse model leads to improvements in heart function and repair. Furthermore, deleting the U2AF1 gene greatly reduces the survival of mice post-MI.
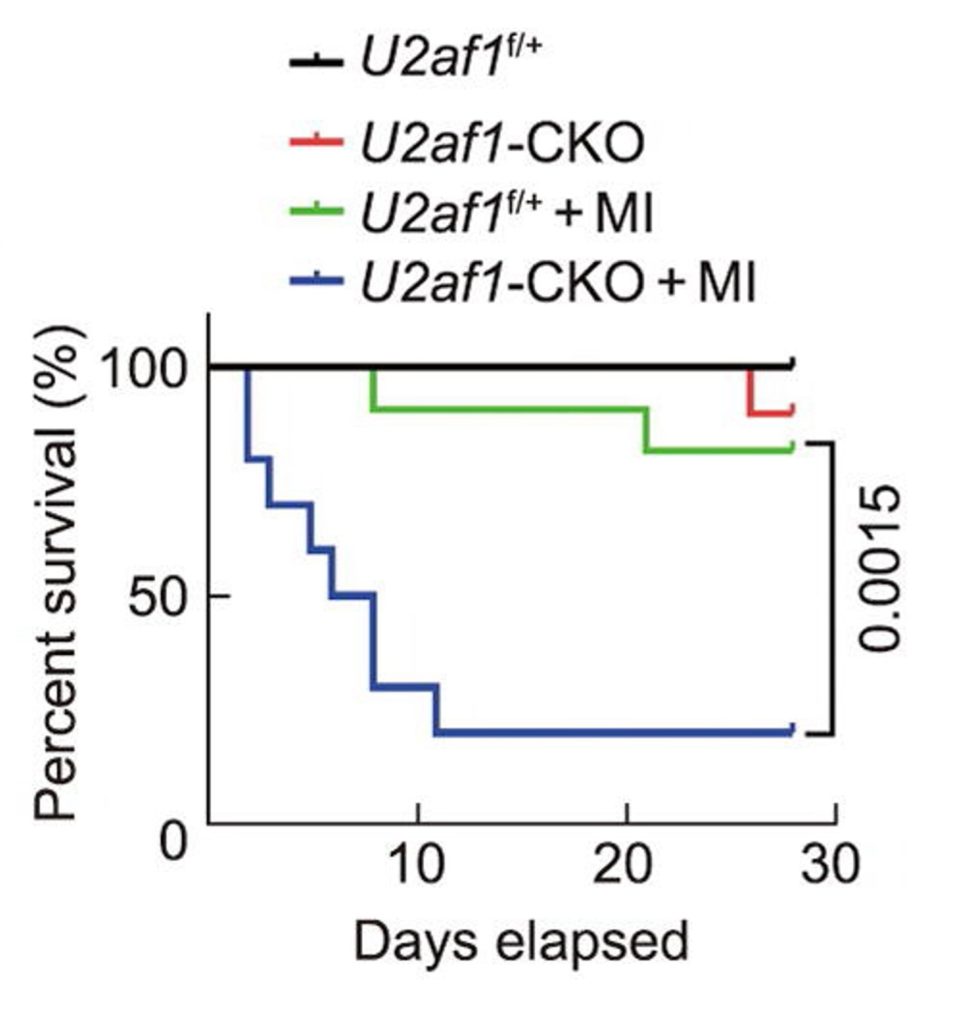
Relevance to Humans
Additional mouse experiments revealed that U2AF1 promotes the formation of new blood vessels, a process called angiogenesis. Importantly, the researchers found that U2AF1 levels were reduced in MI patients, bridging the gap between mice and humans. Furthermore, by measuring angiogenesis levels from the MI patients, the researchers found that higher levels of U2AF1 corresponded to higher levels of angiogenesis. These findings suggest that exosomes from NMN-treated macrophages support heart repair by promoting angiogenesis via U2AF1.
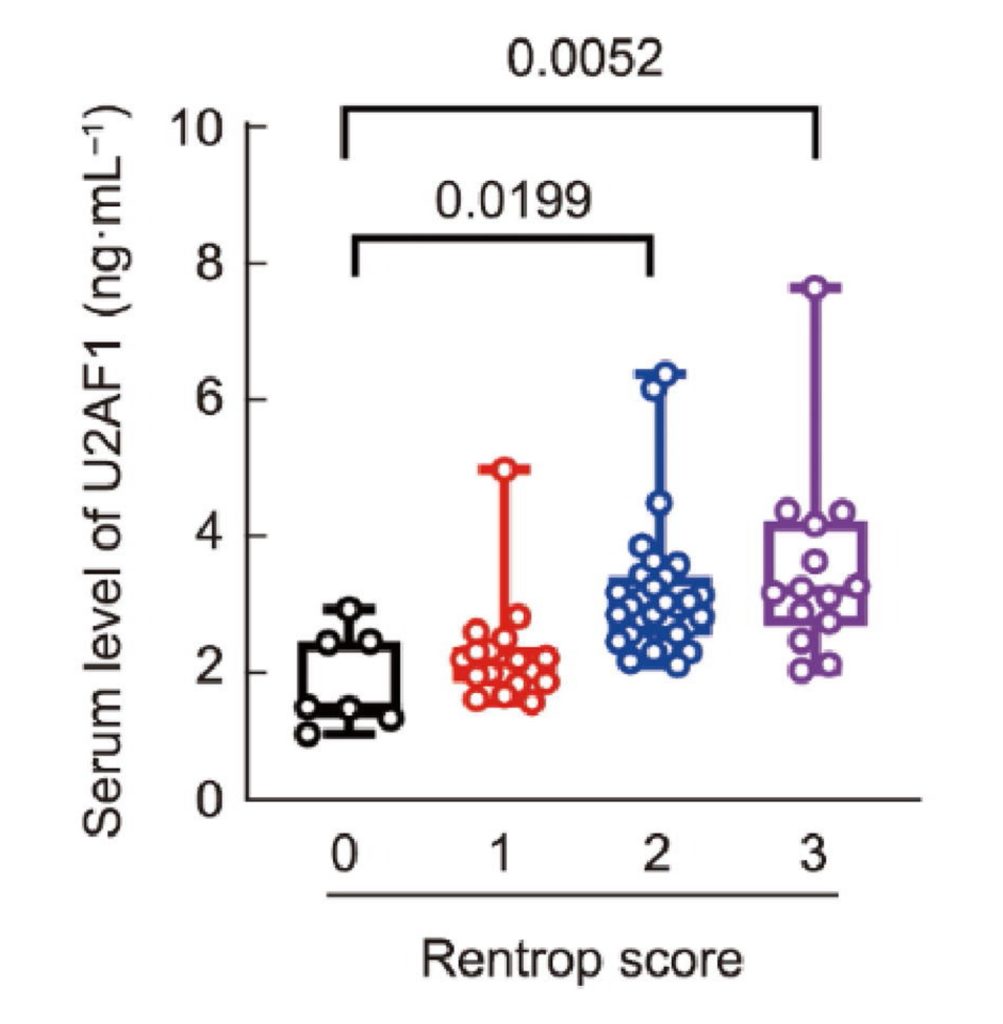
What Does the U2AF1 Protein Do?
U2AF1 is a protein known as a splicing factor. When a gene is transcribed from DNA, a strand of pre-mRNA is formed. The pre-mRNA can be spliced into multiple different mRNA strands that each code for a different variation of a protein from the same gene. The researchers found that U2AF1 alternatively splices the pre-mRNA for a gene called Yap, leading to the translation of a YAP protein isoform involved in cellular proliferation and angiogenesis.
The Future of Heart Repair?
While emergency procedures can often clear the blockage and restore blood flow after MI, the damage to the heart muscle can be permanent. The heart, unlike some other organs, has a limited capacity for self-repair. This is where angiogenesis becomes critical. A robust network of new blood vessels can help bypass damaged areas and resupply oxygen to starved tissues, improving the chances of recovery and reducing long-term complications. NMN appears to promote this recovery by inducing angiogenesis.
However, before initiating clinical trials to test the effects of NMN-based therapies on MI patients, it may be crucial to optimize targeting. Stimulating angiogenesis throughout the entire body without targeting specific sites of damage could potentially support the development of cancer. In order to grow, cancer cells need an adequate supply of blood, which is provided by angiogenesis. Thus, finding ways to direct NMN-based therapies to localized sites in the body, perhaps through the use of exosomes, may be of paramount importance, especially in individuals at risk for cancer, including the elderly.

