NMN Reels Back Metabolic Problems in Obese Mice
Japanese researchers demonstrate that intestinal NAD+ levels are important for maintaining insulin secretion and glucose metabolism.
Highlights:
- Deleting NAMPT – the enzyme that synthesizes NMN – from mouse intestinal cells leads to a muted insulin response and high blood sugar levels.
- Obese mice also exhibit this phenomenon, with stunted NAD+ levels leading to dysfunctional insulin secretion and glucose regulation.
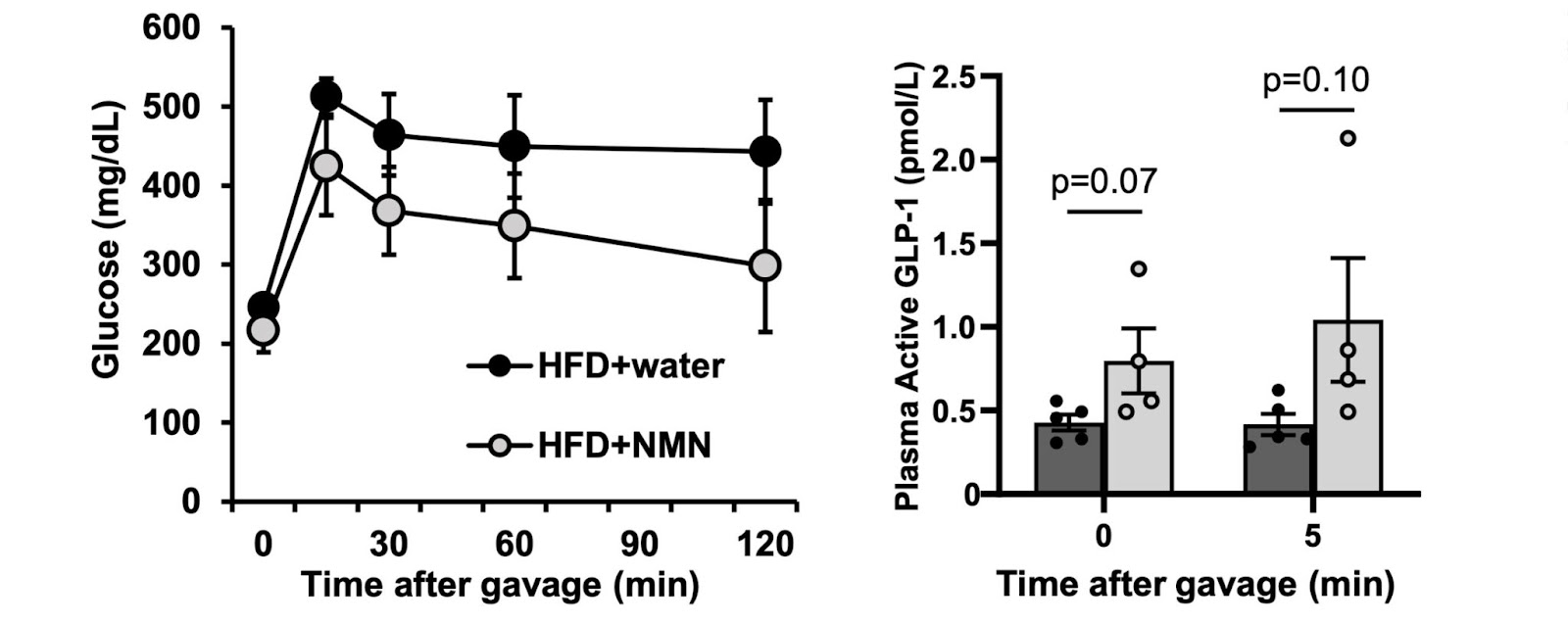
- Adding NMN to the water of these mice reverses the metabolic derangements caused by deleting NAMPT or obesity.
Downing a Slurpee or stuffing our face with candy prompts our intestines to send signals called incretins to tell insulin to mop up all the sugar. Incretins are a group of hormones that are released by intestinal cells in a glucose-dependent manner – more sugar means more incretin release. When incretin regulation goes awry, the resulting faulty insulin secretion and glucose utilization often lead to obesity. Getting a better handle on incretin regulation could have widespread health benefits.
Researchers from Keio University School of Medicine in Japan and Washington University School of Medicine in St. Louis show that NAD+ is essential for intestinal epithelial cells to release one of the most critical incretins, GLP-1 (glucagon-like peptide 1), to regulate insulin secretion and blood glucose levels in response to sugar. Published in Endocrinology, this study shows that intestinal cells in obese mice have diminished NAD+ levels and GLP-1 production, leading to sky-high glucose levels in response to sugar. These results were duplicated in non-obese mice lacking NAMPT – the enzyme synthesizes the NAD+ precursor NMN – in intestinal wall cells. In mice with obesity or intestinal NAMPT deletion, water supplemented with NMN (500 mg/kg/day) restored intestinal NAD+ levels, GLP-1 production, and glucose metabolism in response to sugar.
“Collectively, our study provides mechanistic and therapeutic insights into intestinal NAD + biology related to obesity-associated dysregulation of GLP-1 production and postprandial hyperglycemia,” said Nagahisa and colleagues.
GLP-1 Keeps Glucose Levels In Check
Obesity is associated with high blood sugar levels (hyperglycemia), an important predictor for developing cardiovascular diseases, type 2 diabetes, and nonalcoholic fatty liver disease. In rats fed high-fat diets, the intestinal cells that produce GLP-1 called “L cells” diminish, leading to a decrease in GLP-1 concentration and hyperglycemia in response to food.
In addition, findings from human research imply that changes in the nutrient-induced GLP-1 response are linked to obesity and metabolic dysfunction, such as hyperglycemia. The GLP-1 response, for example, is considerably lower in obese and glucose-intolerant patients compared to healthy-lean subjects, despite contradictory evidence indicating GLP-1 secretion is enhanced or unaffected in obese participants. Diet-induced weight loss, on the other hand, enhances the GLP-1 response and improves hyperglycemia in obese people.
Intestinal NAD+ Biosynthesis Regulates GLP-1 Production And Glucose Metabolism in Mice
Since a previous study recently reported that NAMPT inhibition decreased GLP-1 secretion in cell culture, Nagahisa and colleagues focused on the role of intestinal NAMPT-mediated NAD+ biosynthesis in maintaining intestinal integrity, including incretin production and whole-body glucose metabolism. Their thinking was that intestinal NAD+ plays an essential role in regulating GLP-1 production. So, impaired NAMPT-mediated NAD+ biosynthesis in the intestines could contribute to the development of obesity-induced hyperglycemia.
To test this, Nagahisa and colleagues generated mice with the NAMPT gene deleted from intestinal epithelial cells, which are cells that line the intestinal wall and mediate the absorption and secretion of molecules, including nutrients, water, and hormones. These genetically altered mice displayed diminished GLP-1 production, reduced insulin secretion, and hyperglycemia. In addition, the researchers evaluated changes in intestinal NAD+ biosynthesis mediated by NAMPT in mice with obesity induced by a high-fat diet (HFD). The HFD-fed obese mice had a significant reduction in intestinal NAMPT gene activity and NAD+ levels, accompanied by impaired GLP-1 production and glucose metabolism compared with the lean mice.
Finally, NMN administration restored intestinal NAD+ levels and obesity-associated metabolic derangements, manifested by increased GLP-1 production and decreased hyperglycemia in the genetically modified and diet-induced obese mice. Collectively, these data reveal the crucial role of intestinal NAD+ biosynthesis mediated by NAMPT in the maintenance of GLP-1 production and normal glucose regulation, along with the therapeutic potential of NMN for these obesity-associated metabolic abnormalities.
Clinical Trials On NMN and Glucose Metabolism
Interestingly, it has also been reported that NMN can directly elicit insulin secretion directly in the pancreas to improve glucose metabolism independent of GLP-1. For example, injection of NMN increased insulin secretion in response to high glucose intake in HFD- and aged-induced diabetic mice. In addition, NMN stimulated insulin secretion from incretin-independent islet cells in the pancreas. Taken together, these findings highlight the promise of NMN in improving glucose metabolism following meal consumption because it affects both incretin-dependent and independent pathways.
Moreover, in contrast to oral NMN intake on GLP-1 production in mice, in another study by Dollerup and colleagues, oral administration of nicotinamide riboside (NR), another NAD+ intermediate, did not affect GLP-1 secretion in mice and non-diabetic people with obesity. Mechanisms explaining the difference in GLP-1 production between NMN and NR, two key NAD+ intermediates, remain unclear but may involve the presence of the NMN transporter called Slc12a8, which is expressed at high levels in the small intestine.
For these reasons, Nagahisa and colleagues speculate that oral intake of NMN, but not of NR, enhances intestinal NAD+ synthesis via the NMN transporter in the small intestine, resulting in GLP-1 production. These studies pave the way for further investigating the effect of NMN on insulin secretion and glucose metabolism in humans, which could have widespread consequences for people at risk of cardiovascular diseases, type 2 diabetes, and nonalcoholic fatty liver disease.
Currently, colleagues of one of the authors Jun Yoshino at Washington University School of Medicine in St. Louis, Missouri, are recruiting for a clinical trial testing the effect of NMN (300 mg/day for 16 weeks) on key cardiovascular and metabolic functions, specifically those that are important risk factors for diabetes and cardiovascular disease. This trial will perform similar tests to the ones presented in this paper, like blood glucose measurements after high glucose intake. The estimated completion date for the trial is set for the fall of 2025. This NMN dose is on the lower end of what has been shown to be safe and tolerable in humans, but it still has the potential to move the needle on our understanding of whether NMN has significant health benefits in humans.

