NMN Stimulates Bone Marrow Stem Cells to Form More Bone and Less Fat
Findings reveal a potential connection between NMN treatment and remedy in osteoporosis and aging.
Two hundred and six bones prop up the framework of an adult human, protecting our vital organs, supporting our weight, and enabling our mobility. Inside the rigid surface layer of the organ are honeycomb-like holes. Our bone tissues break down and get continuously replenished. But as we get older, new bone formation can’t keep up with old bone removal. The outer layer becomes thinner and the tiny holes grow larger — the once sturdy bone begins to fall apart inside out.
Chinese scientists from Sun Yat-Sen University discovered that the molecule nicotinamide mononucleotide (NMN) could promote bone generation in mice by stimulating a group of stem cells to differentiate into bone cells instead of fat. The method published in Cell Death & Disease reveals NMN as a novel potential intervention for osteoporosis.
“Our findings reveal a potential connection between NMN treatment and remedy in osteoporotic and aging mice,” proposed Song and colleagues.
Bone Marrow Stem Cells Make Many Cell Types
Scientists have known that mesenchymal stromal cells (MSC) in the bone marrow is a reservoir of multipotent stem cells that can differentiate into a variety of cell types, including bone, cartilage, and fat. The stem cells maintain a balance within the bone tissues. However, when bone marrow MSC aberrantly differentiates towards fat instead of bone tissues, it compromises the formation of bones, resulting in skeletal aging and osteoporosis.
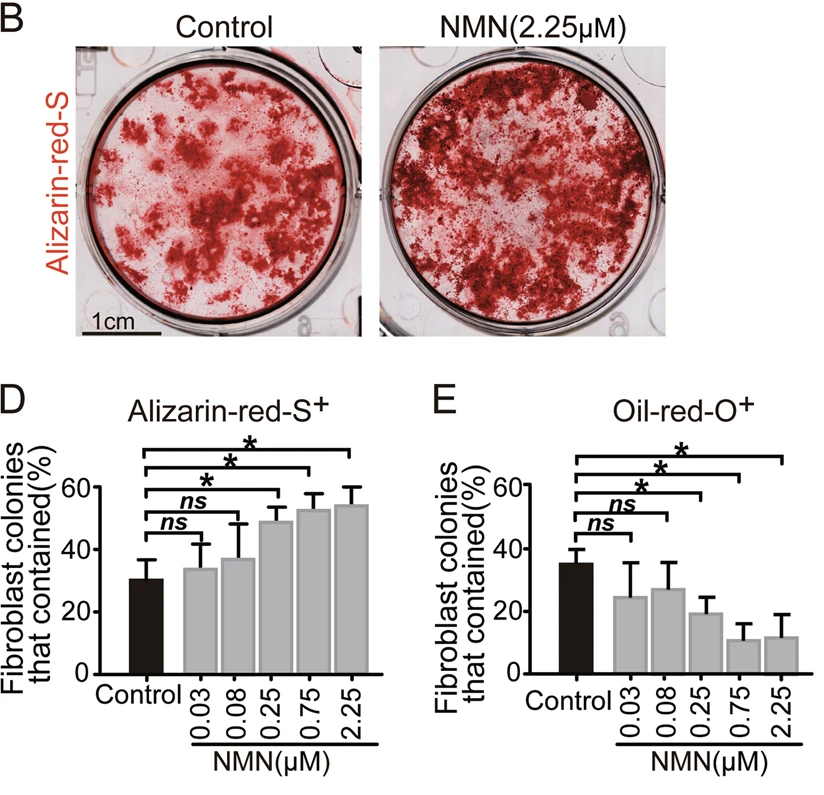
NMN Cultured MSCs Make More Bone and Less Fat
In both cell cultures and adult mice, the research team found that NMN treatment effectively encourages MSC expansion, in which the stem cells scaled up in number. When the researchers further induced the MSC into two different lineages, bone-forming, and fat-forming cells, they found that NMN has a significant and distinct effect on the two lineages.
While bone-forming cell lines treated with NMN produced about 50 percent of osteoblasts, cells that form new bones, the untreated ones only yielded about 30 percent. In contrast, fat-forming cell lines treated with NMN had a lower concentration of adipocytes, which form fat, compared to the untreated cells. The results indicated that NMN promotes the generation of bone while impeding fat formation.
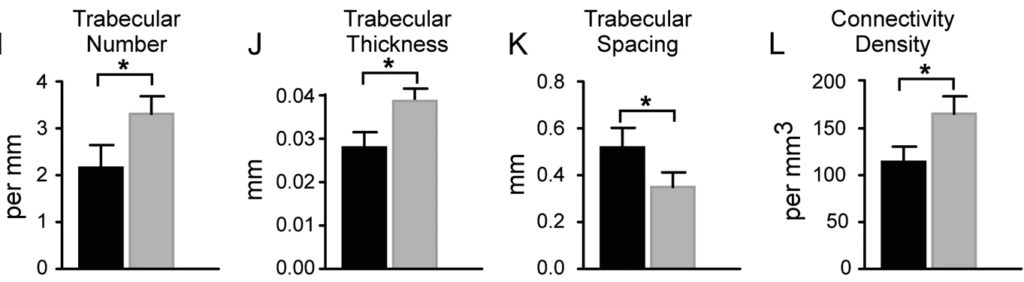
NMN Reverses Age-Associated Bone Loss in Mice
The researchers also observed similar results in aged mice that are a year old. The aged mice that drank the NMN-dissolved water showed an increase in bone density while the number of fat-forming cells decreased compared to normal old mice. However, “NMN does not change bone-fat balance in adult mice,” said the researchers in the study.
In humans, older adults are also more susceptible to osteoporosis. One in every four women over 65 has osteoporosis, while one in every 20 men has it, according to the Centers for Disease Control and Prevention. Osteoporosis is also known as the “silent disease,” not being able to feel the bone grow weaker physically, many won’t even realize they have the disease until they break a bone.
To test out NMN’s therapeutic potential against age-associated bone loss, the team exposed adult mice to irradiation that leads to bone loss and MSC damage, which mimics the osteoporosis effects. The irradiation decreased the MSCs’ ability to expand by 75 percent it also led to fewer osteoblasts but more adipocytes. However, NMN reversed the effects of irradiation. NMN-treated mice were able to expand their MSCs as well as normal mice, their bone density increased, and the intervention effectively inhibited the post-irradiation fat formation by approximately 66 percent.
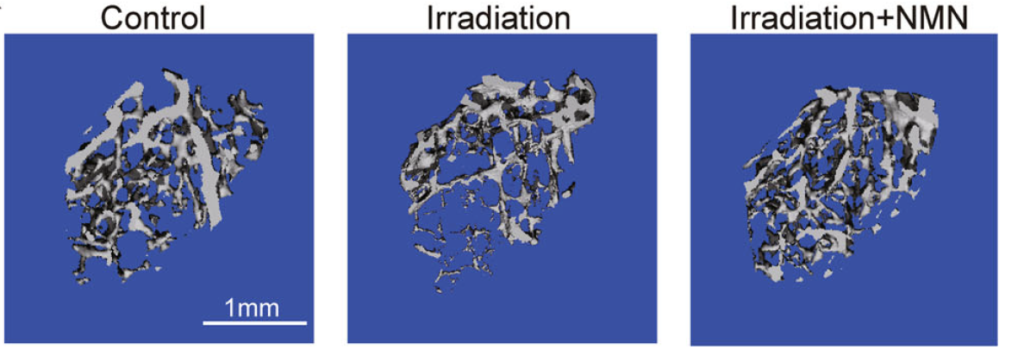
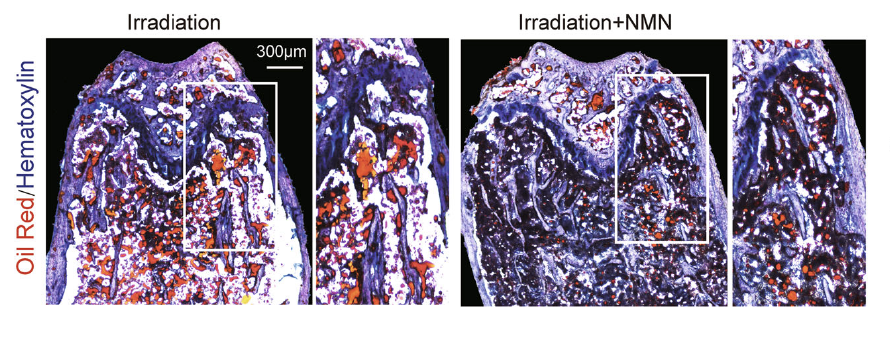
“Our study indicates that NMN enhances the bone-fat balance towards the bone lineage in aged and irradiated mice, suggesting that NMN is a valuable therapy for rescuing bone loss during aging,” noted the authors. “Our study establishes NMN as a promising potential therapy for MSCs expansion and rejuvenation of aged MSCs.”

