NMN Improves Telomere Length and Liver Damage in Mice
Supplementation with nicotinamide mononucleotide (NMN) can preserve telomere length to counteract liver fibrosis
Highlights
-Dysfunction of protective caps at the ends of chromosomes called telomeres is linked to a steep decline in NAD+.
-Increasing NAD+ levels with NMN stabilizes telomeres and improves liver fibrosis.
DNA on ends of chromosomes that shorten with age called telomeres are involved in many conditions, including premature aging. But how exactly this happens and causes age-related disorders to manifest remains uncertain.1
Scientists from multiple institutions, including Baylor College of Medicine, MD Anderson Cancer Center, and the University of Pennsylvania performed experiments on mice showing that nicotinamide mononucleotide (NMN) supplementation ameliorates cellular conditions associated with reduced DNA integrity and counteracts liver disease.
“Our studies demonstrate that NMN stabilize telomeres in a partially Sirt1-dependent manner under conditions of ongoing DNA damage and dampens the DNA damage response,” concluded Amano and colleagues.
Telomeres and liver disease
A well-recognized consequence of compromised telomeres is the increased risk of tissue scarring (fibrosis), with the liver and lung most commonly affected. Liver disease is recognized to occur with a higher frequency in patients with telomere disorders like aplastic anemia and bone marrow failure. Telomere shortening is also a hallmark of long-standing liver disease due to acquired causes such as hepatitis B/C viral infection or alcohol consumption. The accumulation of liver cells with critically short telomeres is associated with disease progression leading to liver cirrhosis, liver failure, and elevated cancer risk.
How are NAD+ levels connected to telomere length and disease?
Sirtuins are a class of NAD(+)-dependent enzymes that impact different cellular processes including DNA repair. Sirtuins are highly implicated in aging and metabolic and age-related disorders.
In the liver, one particular sirtuin called Sirt1 has been particularly well studied among the seven sirtuins and has been shown to play an important role in diverse metabolic processes as well as implicated in the development of liver disease.
Increasing the activity of Sirt1 protects against fatty liver disease and improves insulin resistance induced by a high-fat diet, while lack of Sirt1 in the liver accelerates hepatic steatosis and insulin resistance and is associated with inflammation and oxidative stress.
Telomere Dysfunction Leads to Sirtuin Repression
Since telomere dysfunction and sirtuin repression, independently, are highly associated with predisposition to diseases, accelerated aging, and lifespan reduction, Amano and colleagues examined the interplay between telomere length, sirtuins, and liver disease. To do so, they generated mice with non-functional telomerase, a critical enzyme for maintaining telomeres through cell divisions.
These mice developed premature aging, as the telomeres became progressively shorter, especially in successive generations. These mice displayed traits of telomere dysfunction, such as compromised stem cells, regenerative defects in tissues with high cellular turnover, tissue weakening (atrophy), heart muscle disease (cardiomyopathy), and shortened lifespan.1,2
Compared to non-genetically modified mice, sirtuin levels were reduced in the liver of mice with telomere dysfunction. The degree of repressed sirtuin levels appears to depend upon the degree of telomere dysfunction. In addition to a drop in proteins levels, sirtuin activity decreased.
NMN-Dependent Rescue of Fibrosis and Telomere Maintenance
The repression of sirtuins upon telomere dysfunction would suggest that elevating the activity of several sirtuins would be advantageous based on the protective effect of NAD+ precursors against metabolic disorders, age-related diseases, and stem cell failure. So, Amano and colleagues tested whether NMN could improve the mice lacking telomerase with liver fibrosis. Before injecting the liver with a scarring agent, the researchers treated mice with NMN for two weeks.
They found that NAD+ levels decrease more in mice with telomere dysfunction compared to the non-genetically modified mice, but this could be mitigated with NMN treatment. Interestingly, even in mice deficient for telomerase, NMN treatment was able to improve the length of telomeres.
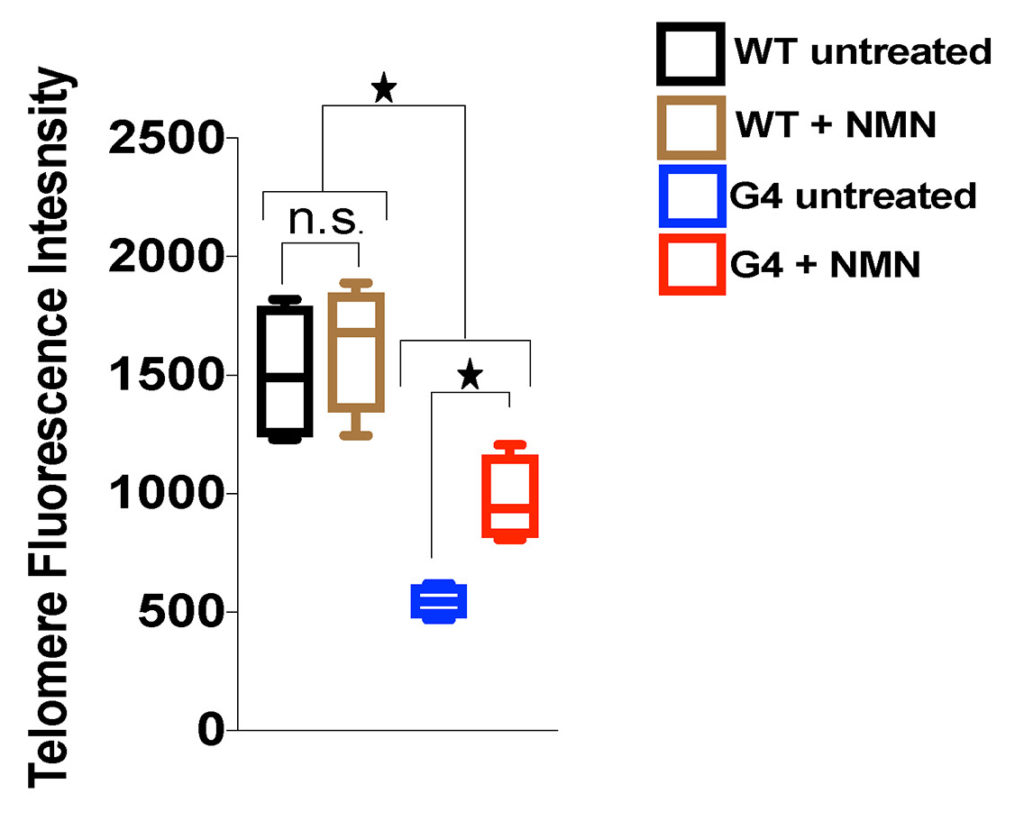
What’s more, NMN reduced the damage and fibrosis of liver cells in telomere dysfunction mice as well as mice without telomere dysfunction. This indicating NMN supplementation protects cells under conditions of increased DNA damage, regardless of telomere status.
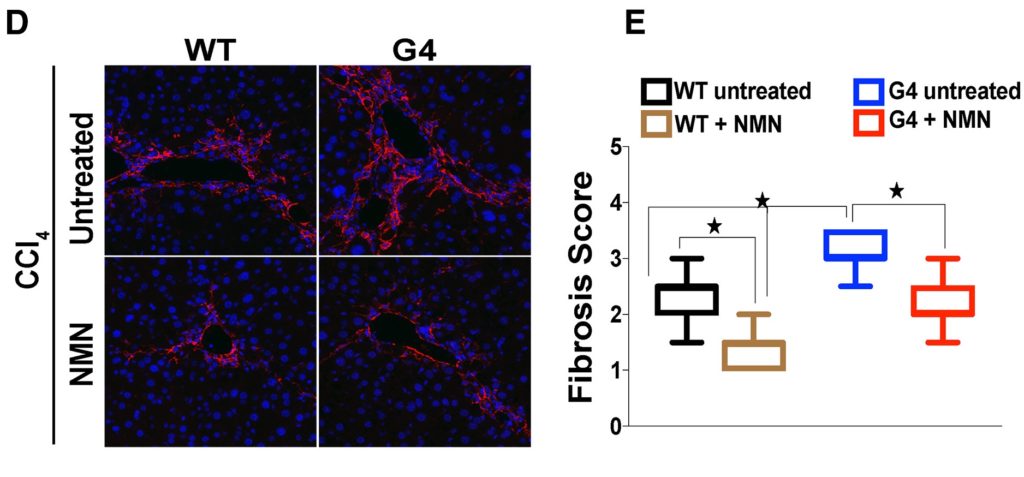
To further interrogate which of the sirtuins is required for the observed effects of NAD+ on telomere length and improvement of fibrosis, Amano and colleagues focused on Sirt1, based on its role in telomere maintenance and because its reduced expression is associated with liver pathologies. To test whether Sirt1 is required for NMN-induced amelioration of liver fibrosis, the researchers induced fibrosis in mice proficient or deficient for Sirt1 in unmodified mice or those lacking telomerase.
They found that the beneficial effects of NMN on liver fibrosis is significantly dependent on Sirt1. To determine whether the effect of NMN on telomere length in mice is Sirt1 dependent, Amano and colleagues analyzed telomere length and telomere integrity in these mice. NMN-treated mice lacking Sirt1 and telomerase had shorter telomeres and poorer telomere integrity compared to mice with intact Sirt1. This indicates a partial requirement of Sirt1 for NMN-dependent telomere maintenance and integrity.
“Our studies show that under conditions of DNA damage, NAD+ levels fall significantly in cells with dysfunctional telomeres and boosting NAD+ levels maintains telomere length in a partial Sirt1-dependent manner and that this ameliorates telomere-dependent liver disease,” concluded amano and colleagues.

