Old Mice That Exchange Blood with Young Mice Have Lower Biological Age and Live Longer, Harvard and Duke Study Shows
Research from Harvard Medical School and Duke University School of Medicine shows that exposure to youthful circulation extends the maximum and average lifespan of old mice.
Highlights:
- For the first time, researchers have demonstrated that exposure to the young circulatory system leads to persistent and systemic slowing of epigenetic aging.
- Notably, this effect correlates with a longer life span and improved physical characteristics.
- While this approach may not be feasible in humans, the analyses methods and insights could translate to the development of anti-aging therapeutics.
As if taken straight from a sci-fi story, researchers found that if you connect the circulatory systems of a young and an old mouse for a period of time and then separate them, the old mouse will live longer and be in better health. According to new research from Harvard Medical School and Duke University School of Medicine, exposing older mice to youthful circulation reduces their biological age, implying a possible reprogramming of the aging process and a promising rejuvenation intervention to slow mammalian aging. The findings were published in the journal Nature Aging.
Real-Life Frankenstein
Researchers have used an unusual method to study circulating factors that regulate the aging process since the 1950s. In this model, known as heterochronic parabiosis (HBP), the circulatory systems of two mice, typically of different ages, are surgically linked together to see how blood from young mice affects blood from old mice and vice versa. This model was critical for establishing the idea that healthy circulation can restore aging tissues’ functions.
HPB has been shown to improve aging characteristics in a variety of tissues, including muscle, liver, heart, brain, and bone. Surprisingly, these effects usually appear after only 4-5 weeks of parabiosis. When blood is exchanged between mice without being sewn together (acute heterochronic blood exchange), similar results are obtained, demonstrating the beneficial effects of ‘young blood’ on the muscle, liver, and brain of elderly recipients. The heterochronic transfer of young blood plasma can also improve the outcomes of age-related diseases, as seen in a mouse model of Alzheimer’s disease.
Despite HPB’s diverse effects on aging cells and tissues, we know very little about the underlying molecular mechanisms. The same can be said about whether the short-term benefits of blood and plasma exchange last after the procedure is completed. Finally, it is unknown whether HPB can delay or reverse epigenetic aging.
Prolonged heterochronic parabiosis decreases biological age and promotes longevity in old mice
The detachment of mice after a period of time is a previously reported but rarely used HPB procedure; typically, the mice remain conjoined until the end of their lives. A recent study discovered the persistence of aged blood stem cells in the young bone marrow niche as a result of HPB months after parabiont surgical separation. However, no studies had investigated longevity or the long-term effects of HPB on health.
This study presents the results of a protracted HPB study and a subsequent period of detachment. Co-authors Bohan Zhang, David E. Lee, and Alexandre Trapp investigate what happens when the circulatory systems of old mice (20 months old) and young mice (3 months old) are connected for 3 months and whether the effects vary depending on the length of attachment. A collaborative effort between the White Lab (Duke University) and the Gladyshev Lab (Harvard University) led to the discovery that old mice separated from young mice had an extended life span, with a 6-week increase in median life span and a 2-week increase in maximum lifespan when compared to old mice tied to other old mice. They also observed improved physical function in the old mice that exchanged blood with young mice, as these older mice were far more active than their counterparts that exchanged blood with other old mice.
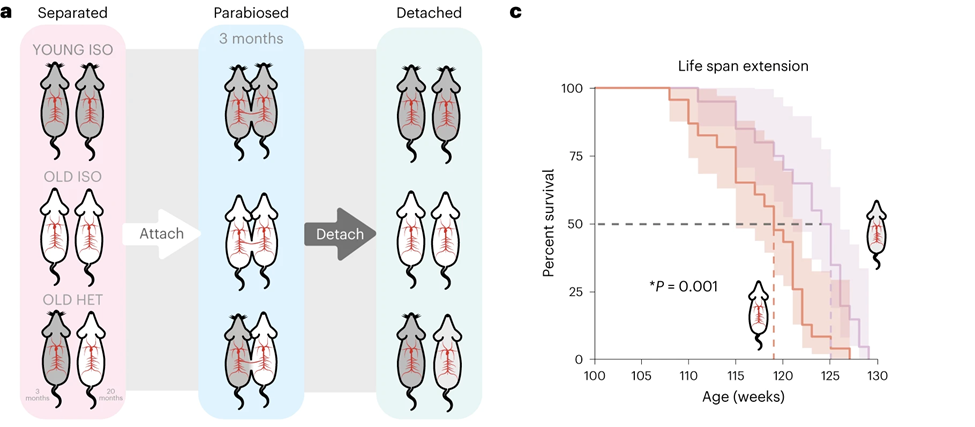
Furthermore, HPB resulted in improvements in a variety of aspects of aging biology. After a 3-month HPB, Zhang, Lee, and Trapp reported a significant decline in biological age (as determined by epigenetic aging) in old mice. This decline persisted even after a 2-month detachment period. The aging clock analyses showed a decrease in epigenetic age of up to 30% from HPB, even though the lifespan extension effect on the detached mice, while still significant, was not as strong.
The rejuvenation effect from three months of HPB was stronger than that observed on the more traditional, short-term HPB, which is 5 weeks. These findings suggest the presence of profound and persisting molecular rejuvenation effects after exposure to young circulation, leading to an extended life span and health span. Importantly, they showed that the rejuvenating effect on gene activity of parabiosis is similar to that of other longevity interventions while being negatively associated with changes induced by aging.
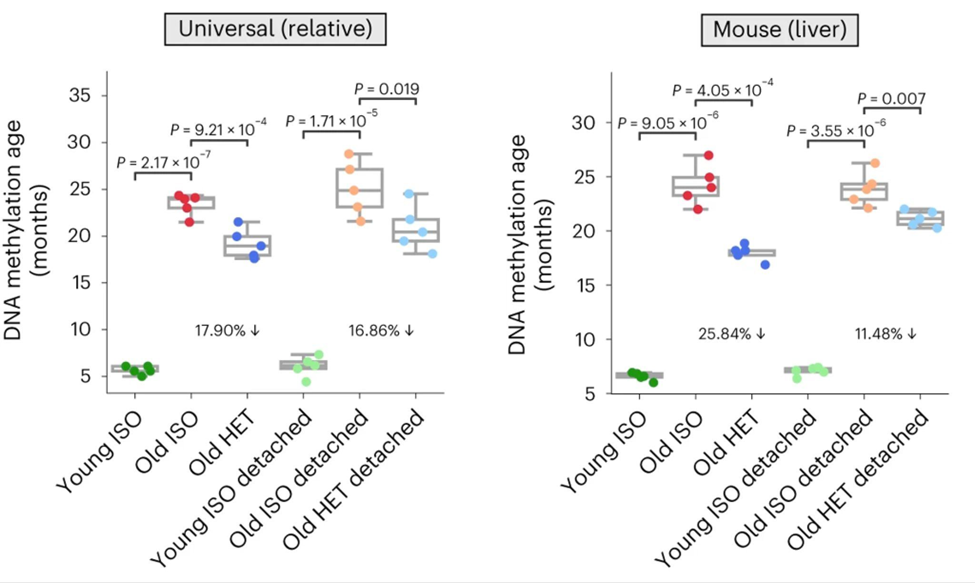
Importantly, Zhang, Lee, and Trapp showed that the molecular effect (that is, the effect on the transcriptome) of parabiosis is similar to that of other longevity interventions while being negatively associated with changes induced by aging. Collectively, we discovered a rejuvenation effect upon long-term heterochronic parabiosis across multiple tissues that is sustained even after a period of detachment.
What’s Feasible, And How Far Would You Go?
While the researchers said that this was an exciting moment, their excitement quickly turned into more questions and the acknowledgment that there is still a lot more work to do. The next challenge is to take a step further and understand the ‘how and why’ of their observations, with the ultimate goal of harnessing and translating these findings to human therapeutics. Along these lines, parabiosis is currently not feasible for human interventions due to logistical concerns, but these results provide support for the possibility that other blood-related strategies, such as the injection of young plasma components or plasma dilution approaches, may have a rejuvenating effect.
Bryan Johnson, a wealthy entrepreneur estimated to be worth $400 million, made headlines when he shared the results of his blood swap with his son. The results were somewhat disappointing, and Johnson announced that he would stop using blood transfusions because they had no anti-aging effects. He added that plasma exchange might be helpful for biologically older populations or for particular conditions, but that it would not be helpful in his case because it would not stack benefits on top of his current interventions.
All of this raises the question: How far would you go to extend your life? HPB may be completely out of the question (unless you can set up some sort of clone farm, which would be unethical in so many ways) because I am not sure what young person would permit someone older than them to become conjoined with them. Perhaps the closest we will ever get is the transplantation of bodily components, like bone marrow, which contains blood stem cells, into elderly people. We will have to wait and see what the next Frankenstine idea turns out to be.

