Precision Nutrigeroscience: Using Individualized Dietary Treatments to Slow Aging
Buck Institute researchers propose developing anti-aging plans based on analyzing specific age-related biomarkers of an individual to optimize diet-based therapeutics.
Highlights
· Nutrient restriction promotes health and counteracts age-related morbidities, but, given the difficulty to restrict nutrients in humans, efforts are needed to identify compounds or behavioral interventions that induce or mimic dietary restriction.
· A viable and seemingly promising approach is to optimize strategies that combine moderate dietary restriction with therapeutic and behavioral interventions like exercise that promote health and longevity.
· Buck Institute researchers propose developing biomarker panels for measuring biological age, determining how dietary interventions alter biomarkers within individuals, and optimizing these interventions to improve age-related health outcomes.
To some researchers in the field of aging (geroscience), dietary restriction is the most robust means to extend lifespan and healthspan — the number of years lived in good health without illness — without introducing genetic modifications. Many aging-associated mechanisms are nutrient responsive, but despite the pervasive functions of these pathways, the benefits of dietary restriction often vary among individuals and even among tissues within an individual.
In the journal Cell Metabolism, Wilson and colleagues from the Buck Institute propose a framework called precision nutritional geroscience, or nutrigeroscience for short, which would utilize individualized treatments and predict outcomes using biomarkers based on genetics, sex, tissue, and age. “Everyone is hoping for a ‘one size fits all’ intervention when it comes to diet, and work in our lab and several others show that this is simply not going to be the case,” says Buck professor Pankaj Kapahi, Ph.D., the article’s senior author, whose team aims to understand the mechanisms by which nutrient signaling and metabolism influence aging and age-related diseases.
Evaluating the Beneficial Effects of Calorie Restriction
In 1935, Dr. Clive McCay showed that dietary restriction in the form of calorie reduction without malnutrition prolongs the average and maximal lifespan in rats compared with those allowed to eat freely. Four years later, his subsequent experiments showed that mice consuming about 40% of daily calories had roughly a 50% lifespan extension.
The potential of calorie reduction to extend lifespan has created a prominent subfield of aging research that promises a simple but elegant method to enhance lifespan and healthspan, the period of life in which an individual is healthy, with minimal side effects. Calorie reduction is now known to extend lifespan in nearly thirty species, including nonhuman primates, and remains the most robust way to extend lifespan in the laboratory.
In humans, a two-year clinical study on the effects of chronic, moderate calorie reduction showed a slowing in metabolism and decreased production of free radicals. Though this study indicated that changes induced by calorie reduction reduced markers of aging, the long-term effects of calorie reduction in humans remain unproven.
We do know that short-term calorie restriction in humans induces the formation of “efficient mitochondria” in skeletal muscle. Healthy, nonobese human adults undergoing 25% calorie restriction for up to 2 years also show reduced proinflammatory markers. Calorie restriction is reported to delay age-related cognitive decline in complex mammals including humans.
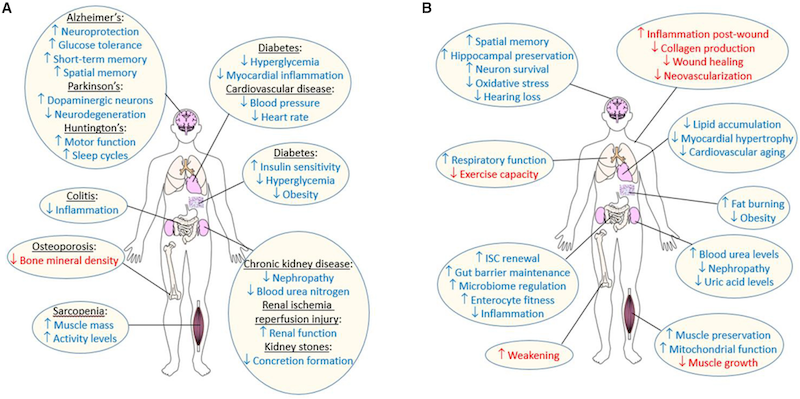
Distinct from calorie reduction, dietary restriction encompasses all dietary interventions that restrict the intake of specific nutritional components. Thus, dietary restriction incorporates calorie reduction and limitation of macronutrients like amino acids, protein, or glucose (sugar) and may include varying fasting intervals. Despite changing dietary restriction protocols, almost all dietary interventions have been reported to influence lifespan in at least one model organism and largely influence similar cellular pathways.
In humans, the role of dietary macronutrients in mortality is complex. Generally, with carbohydrates, mortality risk is U-shaped, meaning that low and high amounts of carbs are more highly associated with mortality than moderate amounts. With fat, the consumption of animal fats leads to higher mortality and consumption of plant-based fats leads to lower mortality.
But genetics can drive the variability in the response to dietary restriction. Given the variation across populations, individuals, and tissues, it is reasonable to expect an array of responses to different regimens. Further, the means of assessing aging vary significantly across studies, adding to difficulties in determining how dietary restriction benefits longevity and health. Despite these variations in methodologies and responses, the knowledge of pathways that mediate nutrient signaling remains critical to help translate findings to humans.
The Need for Precision Nutrigeroscience
Kenneth Wilson, the first author of the editorial, thinks that it’s essential that everyone in the field understand and appreciate that there are many different ways to look at what an intervention might be doing.
“You might see a response in one case, but that response might not work in another strain of the same species,” says Wilson. “Conversely, if you’re not seeing something, that doesn’t mean that the intervention you’re testing doesn’t work; it just means you’re not using the right model for that intervention.”
Wilson proposes that there are likely effective therapeutics that already exist that have been discounted because they haven’t tested well in a particular strain or species of a model organism. “There is a real benefit in giving up the search for a ‘one-size-fits-all’/’magic pill’ solution to the physical problems of aging,” he says. To illustrate, Wilson proposes that the person’s age utilizing dietary restriction is also a factor that needs to be studied and considered. It’s been shown that people who are older may not want to limit their dietary intake, especially when it comes to protein which helps preserve muscle mass.
Creating a Precision Nutritional Geroscience Framework
To create the new framework of precision nutrigeroscience, Kapahi says that he’s had to change how he looks at aging. “A lot of us get very deep into the minutia of understanding what I call two-dimensional biological pathways, and we are forgetting a whole other dimension which is that these pathways are different in each individual and within tissues in individual bodies. Context matters, and we can’t make real progress in this field without including that. We will end up hurting the field because eventually, interventions won’t work, and then people will get disappointed.”
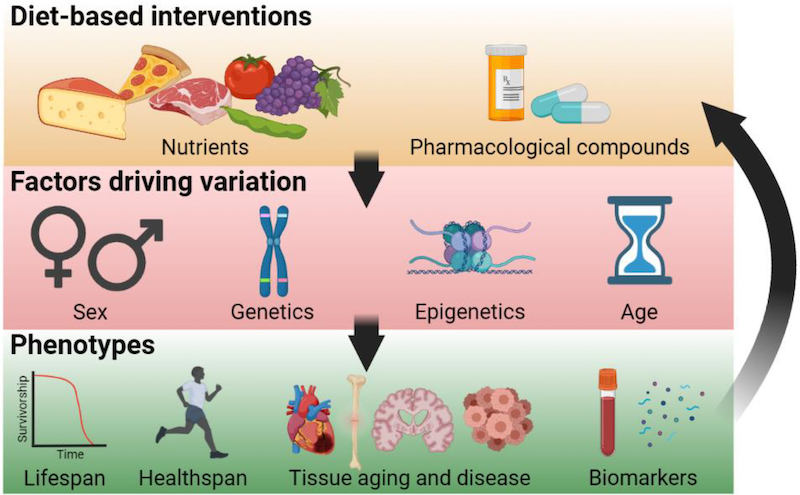
“We know that the science is complex, but if we can frame dietary restriction within the context of precision medicine, everything that people are learning in the field will apply to the larger effort, which is all about improving and extending human health,” says Kapahi. “Bottom line is that there are no ‘negative’ or ‘positive’ data – we can glean insights from all of it, and it will take a large team effort to fully develop Precision Nutrigeroscience.”
Kapahi says that the goal of precision nutrigeroscience is to give scientists who are entering the field of aging research an overview to start separating the forest from the trees when it comes to dietary restriction. He also points out that it is important to understand the exceptions to the current paradigm that dietary restriction is beneficial across the board in every species studied while providing light at the end of the tunnel.

