Researchers Unveil “Anti-aging” Vaccine in Mice
A study published in Nature Aging suggests that vaccination against toxic, non-replicating cells could potentially prevent aging and age-related diseases.
Highlights
- A cell surface molecule called Gpnmb is enriched in specific senescent cells in humans and mice.
- Deletion of Gpnmb-positive cells improved systemic metabolic abnormalities and reduced atherosclerosis in mice fed a high-fat diet.
- Senolytic vaccination also improved typical and disease-like characteristics associated with aging and extended the male lifespan of mice with an accelerated aging disorder (progeroid mice).
The action of making a person or animal immune to infection (immunization) has come a long way since the debut of vaccines in the late 18th century — when Dr. Edward Jenner collected bits of smallpox from the arm of a milkmaid named Sarah Nelmes and scratched it into the arm of an 8-year-old boy — to today’s SARS-CoV-2 injection. Scientific giants including Louis Pasteur and Jonas Salk have helped humans escape the wrath of rabies, anthrax, and polio, rendering these viruses essentially non-threatening. But can vaccines directly target and eliminate age-related processes and diseases?
In an article published in Nature Aging, Suda and colleagues demonstrated that a vaccine eliminating senescent cells related to aging and age-related disease ameliorated aging in aged mice and prolonged the lifespan of mice with premature aging. The Japanese research team from Juntendo University Graduate School of Medicine and Niigata University Graduate School of Medical and Dental Sciences show that vaccination against senescent cells could improve atherosclerosis and metabolic dysfunction in mice.
“We can expect that (the vaccine) will be applied to the treatment of arterial stiffening, diabetes, and other aging-related diseases,” Juntendo University professor Toru Minamino said.
How can senescent cells be eliminated to improve aging and longevity?
Senescent cells accumulate in various tissues with aging or in response to metabolic stress. For example, senescent cells that line blood vessels called vascular endothelial cells are observed in human atherosclerotic plaque. Senescent vascular endothelial cells exhibit functional abnormalities, such as impairment of blood vessel relaxation, and these cells also produce inflammatory molecules known as senescence-associated secretory phenotype (SASP) factors. Both of these changes accelerate the development of atherosclerosis. Visceral adipose tissue develops senescence-like features in patients with type 2 diabetes and promotes insulin resistance.
Several agents that eliminate senolytic cells, also known as senolytics, improve age-associated pathologies and diseases reversibly and extend the healthy lifespan in aged mice. While senolytic therapy has progressed, concerns remain regarding the safety and specificity of the senolytics reported on to date. But not all senescent cells contribute to aging and disease; some are useful and contribute to tissue repair. So, more cell or tissue-specific treatments that efficiently eliminate unwanted senescent cells without affecting the beneficial subset of these cells are highly sought after.
Elimination of Gpnmb-positive cells attenuates metabolic abnormalities and atherosclerosis in mice fed a high-fat diet
To develop a more specific senolytic therapy with fewer off-target effects, Suda and colleagues adopted an approach targeting antigens — molecules that antibodies can bind — specifically expressed by senescent cells. The Japanese research team identified a cell surface protein Gpnmb (glycoprotein nonmetastatic melanoma protein B) as a molecular target for senolytic therapy. Gpnmb levels were upregulated in vascular endothelial cells and leukocytes of patients and mice with atherosclerosis.
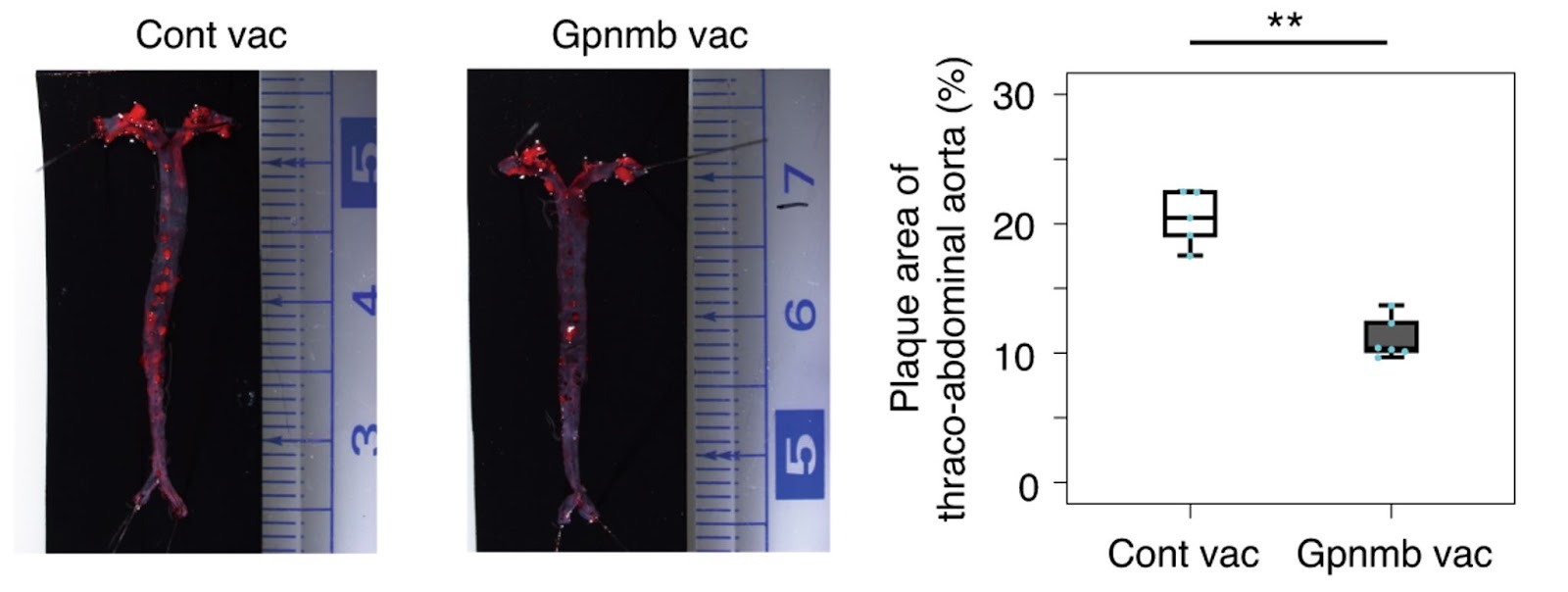
To prove that Gpnmb could be a target for senolytic therapy, Suda and colleagues genetically ablated cells positive for Gpnmb. Deletion of Gpnmb-positive cells attenuated senescence in adipose tissue. What’s more, elimination of these cells in mice fed a high-fat diet improved systemic metabolic abnormalities and reduced atherosclerotic burden.
Gpnmb vaccination decreases tissue senescence and alleviates typical and disease-like age-related characteristics.
Suda and colleagues then sought to develop a peptide vaccine for Gpnmb using bits of the protein. Immunized mice against Gpnmb had reduced senescent cells. Suda and colleagues demonstrated that eliminating Gpnmb-positive cells by vaccination could improve mice’s high-fat-diet-induced atherogenesis and metabolic dysfunction.
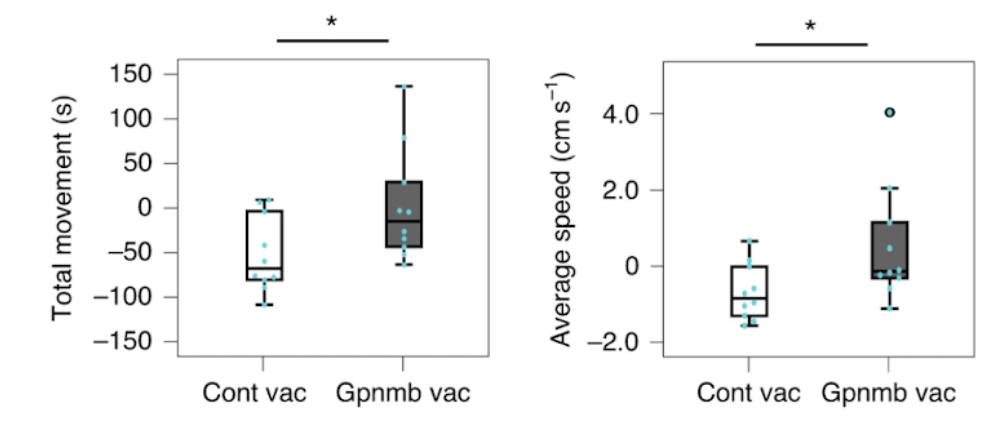
To investigate the effect of vaccination on normal aging, Suda and colleagues administered the Gpnmb vaccine to middle-aged mice (50 weeks old) and examined their performance in the open field test before vaccination and 20 weeks after vaccination (70 weeks old). In the control group, both total movements and the average movement speed decreased with age, but these age-associated changes were significantly ameliorated by Gpnmb vaccination.
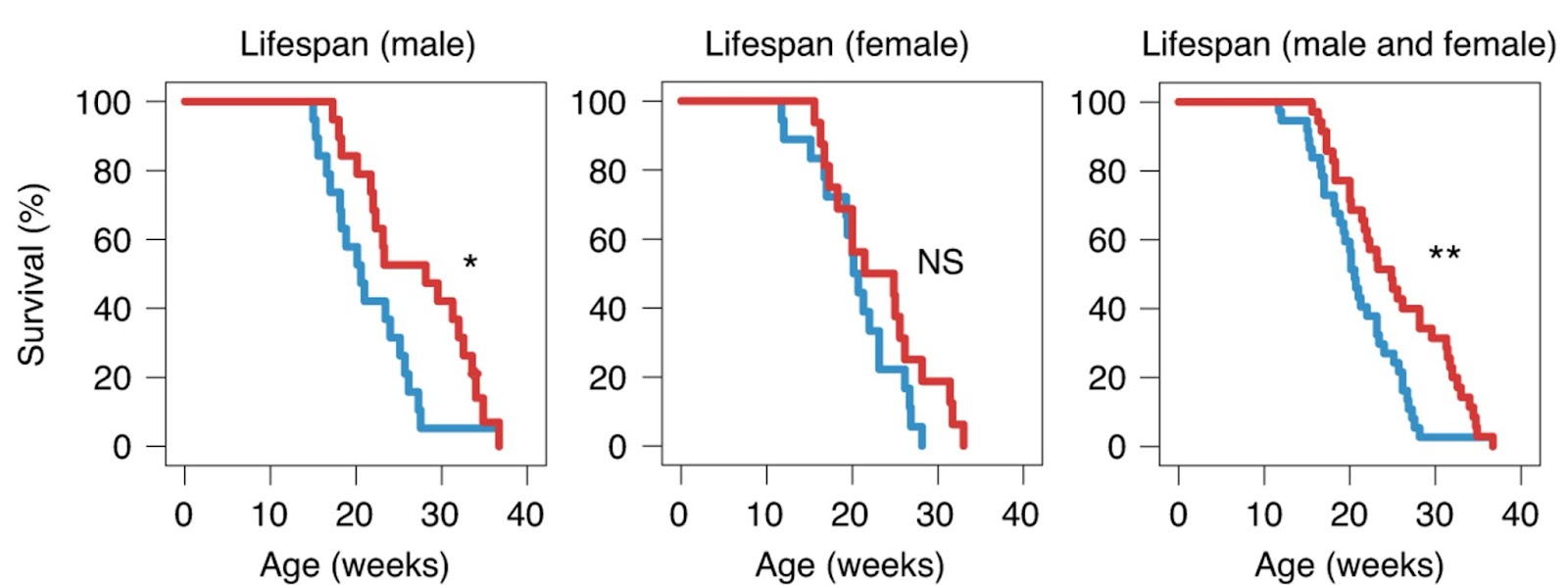
To investigate the effects of the Gpnmb vaccine on the lifespan, Suda and colleagues vaccinated mice that modeled Hutchinson–Gilford progeria syndrome — the premature aging disease — at ten weeks of age and evaluated their survival. In the control group, all mice died by 30 weeks of age. In contrast, mice, especially male mice, administered the Gpnmb vaccine showed a better survival rate, even when administered at ten weeks of age. Likewise, administration of the Gpnmb vaccine significantly extended the median lifespan of progeroid mice, especially male mice, compared with mice treated with the control vaccine.
The current study also compared the effects of Gpnmb vaccination with several senolytics, including the combination of dasatinib and quercetin as well as Navitoclax. Notably, the dasatinib plus quercetin and Navitoclax groups improved the senescence-like changes in fat tissue at 16 weeks, but these beneficial effects declined at 24 weeks. In contrast, Gpnmb vaccination consistently improved senescence-like phenotypic changes in visceral adipose tissue. While dasatinib and quercetin or Navitoclax improved glucose intolerance, insulin resistance developed at 24 weeks of age. In contrast, the beneficial effects of Gpnmb vaccination on glucose metabolism were sustained until 24 weeks of age. Finally, Navitoclax treatment significantly reduced white blood cell and platelet counts and prolonged bleeding time.
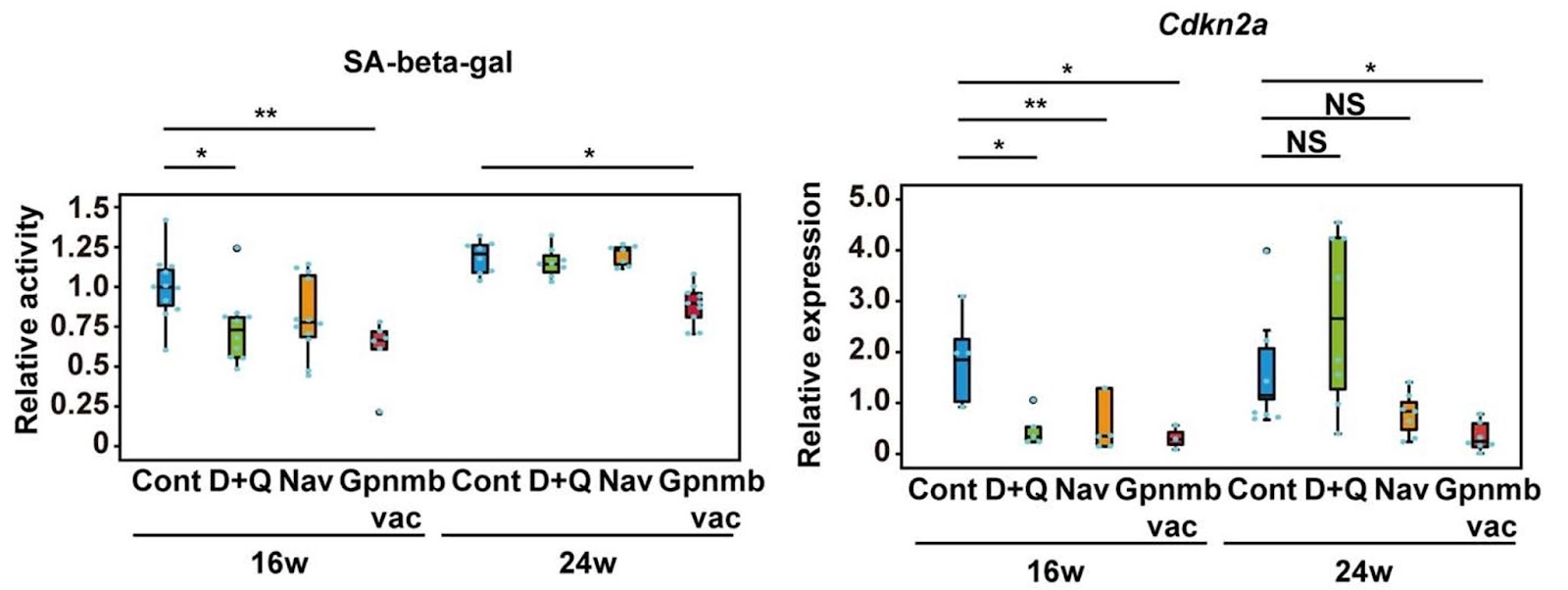
These findings show that, compared to known senolytic compounds, a senolytic vaccine may be the better option.This study shows that vaccination targeting Gpnmb could be a potential strategy for new senolytic therapies, especially for the treatment of metabolic dysfunction and atherosclerosis.
Okay, this is a big deal: Vaccinations against aging work. Sure, it’s a proof-of-principle study in a rapidly aging mouse, but there’s little doubt this will be possible in us one day. Link👇1/3 pic.twitter.com/79NNcXCGZZ
— David A. Sinclair (@davidasinclair) December 11, 2021
It will be interesting to see if different cell surface molecules can be identified in senescent cells from different cell types so that these senolytic vaccines can directly target age-related diseases in certain tissues. Perhaps senolytic vaccines will be the answer to treating elusive age-related diseases, particularly neurodegenerative diseases like Alzheimer’s.