Scientists Engineer Breakthrough Injection That May Reverse Alzheimer’s Disease
Within two hours after the injection, mice exhibited a nearly 45% reduction of toxic, Alzheimer’s disease-associated plaques in the brain.
Highlights
- The injection contained tiny, artificial vesicles coated with short chains of protein building blocks that act as keys to restore the function of a brain plaque-clearing protein called LRP1.
- Giving the injection to mice with an aggressive form of Alzheimer’s disease restored their cognitive faculties, similar to those seen in non-treated mice without Alzheimer’s pathology.
- The cognition-restoring properties of the injection lasted at least six months after the initial treatment.
For decades, figuring out how to treat Alzheimer’s disease has been an insurmountable conundrum for researchers. Starting in the 1980s, researchers began developing drugs that target toxic amyloid-beta protein plaques in the brain, a hallmark of Alzheimer’s disease, in an attempt to address this devastating age-associated disease. However, results from drugs targeting these plaques directly have been frustratingly limited.
Now, in a landmark new publication in Signal Transduction and Targeted Therapy, Battaglia and colleagues from University College London present groundbreaking data that flips the script on counteracting Alzheimer’s disease. Instead of attacking brain plaques, the researchers restored the brain’s self-cleaning system, giving it what it needs to clear Alzheimer’s-associated plaques.
Along these lines, injecting tiny nanoparticles called polymersomes into mice with advanced Alzheimer’s cleared nearly half of the Alzheimer’s-associated amyloid plaques within two hours. In addition to these striking results, the injection restored spatial learning and memory in the mice modeling Alzheimer’s, comparable to their healthy peers without Alzheimer’s pathology. Importantly, the restoration of cognitive faculties lasted for at least six months after a single treatment. These findings reveal a potential new way of addressing Alzheimer’s disease with nanoparticle therapeutics, through the restoration of the brain’s natural waste clearance mechanism.
An Explanation of the Brain’s Waste Disposal Mechanism and the Blood-Brain Barrier
To get a grasp on how these nanoparticle injections work, some background information on the blood-brain barrier needs to be explained. In that regard, the brain is an extremely sensitive organ and needs a way to ensure toxins and pathogens do not enter it. This is where the blood-brain barrier comes in, and it serves the brain and nervous system as a semipermeable membrane separating the blood in circulation from neural tissue. In that sense, the blood-brain barrier is similar to a bouncer that checks the ID of every molecule, only allowing nutrients to enter the brain and nervous tissue.
All the same, the blood-brain barrier’s role as a kind of bouncer is not its only job. It also disposes of waste, sort of like taking out the trash.
Furthermore, in its full functional capacity, under optimal physiological conditions, the blood-brain barrier helps clear harmful amyloid-beta, a sticky protein produced as a normal aspect of brain function. However, in Alzheimer’s disease, the amyloid-beta clearance process breaks down and becomes dysfunctional. In this circumstance, amyloid-beta builds up, forms clumps, and eventually wreaks havoc on cognition, along with causing inflammation, disrupting neuronal communication, and ultimately leading to the death of neurons.
In the past, scientists saw the breakdown of the blood-brain barrier as a phenomenon happening during the latter stages of Alzheimer’s disease, occurring as a consequence of this condition. However, a growing body of evidence suggests that this way of thinking may be backward. In fact, the dysfunction of the blood-brain barrier could actually be one of the first things to go wrong in Alzheimer’s disease brain pathology. In that sense, the breakdown of the blood-brain barrier could be an early event that triggers the whole catastrophic cascade of Alzheimer’s pathology.
“Most current Alzheimer’s treatments try to remove amyloid-β or protect neurons once damage has already occurred,” says study author Giuseppe Battaglia in a press release. “But by that stage, the brain’s protective barrier is already breaking down; it can no longer deliver nutrients or clear waste properly. We focus on repairing this barrier because it sits at the root of the problem. A healthy [blood-brain barrier] supports brain cells, regulates inflammation, and maintains the environment neurons need to function. By restoring the vasculature, we help the brain recover its natural balance, making any other therapy more effective and lasting.”
An Injection of the Nanoparticle Polymersomes Restores the Blood-Brain Barrier
Restoring the functional capacity of the blood-brain barrier and its waste disposal mechanism serves as a difficult task. As such, the brain contains about 100 billion capillaries, most of which are components of the blood-brain barrier. This means that a phenomenal number of these blood vessels are a part of a complex, highly selective barrier and waste disposal system.
Accordingly, a key player in this system’s waste disposal apparatus is a protein called LRP1, with a key role in clearing toxic beta-amyloid proteins. Interestingly, in Alzheimer’s disease patients, production of LRP1 declines dramatically. Furthermore, the reduced number of LRP1 proteins remaining becomes engulfed by the sticky amyloid-beta proteins, and this process winds up destroying many remaining LRP1 proteins.
To tackle the reduced number of LRP1 proteins, which help to clear amyloid-beta proteins, Battaglia and colleagues engineered a nanoparticle delivery system—a tiny, synthetic sphere called a polymersome. These polymersomes act as a drug, with minuscule components that work together and bind with a mid-level affinity to LRP1, which keeps LRP1 from being degraded and helps it to function properly. Along those lines, too much binding affinity, as occurs with amyloid-beta proteins, and LRP1 gets broken down. Too little binding affinity, and the polyersomes have little effect. As such, the mid-level binding affinity of the polymersomes’ components hits a sort of sweet spot that keeps the LRP1 proteins’ waste clearance faculties fully functional.
“Instead of using a single active molecule like traditional drugs, our nanoparticles are composed of many small components that assemble, much like building blocks,” says Battaglia. “These components work collectively, not just to deliver a drug, but to interact intelligently with the blood-brain barrier. That’s why we call them “supramolecular drugs”: they act through structure and communication, helping the brain’s own cells restart processes that have stopped working in Alzheimer’s disease.”
Battaglia and colleagues covered the surface of the polymersome spheres with special chains of protein building blocks, called peptides. To achieve the so-called sweet spot of binding affinity, the researchers added 40 special peptides to the surfaces of the polymersomes. Accordingly, too few peptides would result in less binding and lower efficacy, and too many peptides would mimic the effects of amyloid-beta plaques, binding too tightly.
Battaglia and colleagues then tested how a single injection of the polymersome nanoparticles affected mice suffering from brain amyloid-beta buildup. To do this, the research team took 12-month-old mice that were genetically engineered to develop an aggressive, Alzheimer’s-like condition. These mice were roughly the equivalent age of 44-year-old humans, and their brains were plagued with amyloid-beta plaques. Moreover, these mice displayed significant cognitive impairment.
Intriguingly, within two hours of receiving one injection of the polymersome nanoparticles, the concentration of brain amyloid-beta proteins plummeted nearly 45%. Not only did the concentration of toxic amyloid-beta plaques decrease in the brain, but amyloid-beta levels also increased in blood plasma. This occurrence served as a smoking gun: the toxic plaque was being cleared from the brain and pumped into circulation for disposal. In line with this notion, the amount of amyloid-beta cleared from the brain almost perfectly matched the surplus found in the blood plasma. These findings support that Battaglia and colleagues’ polymersomes quickly and efficiently helped restore LRP1’s waste disposal properties to clear amyloid-beta plaques from the brain in mice.
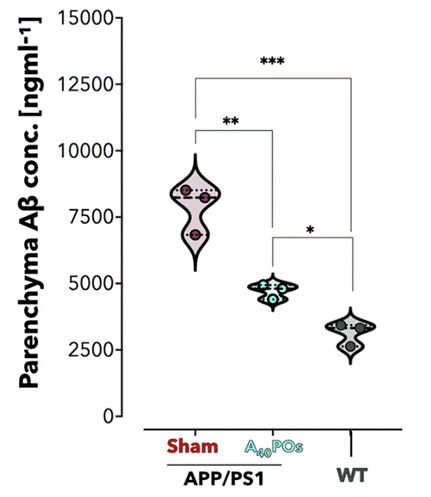
Importantly, the polymersome injection restored spatial learning and memory performance in mice with the aggressive Alzheimer’s-like condition. Astonishingly, six months after that one injection, the improved cognitive faculties of these mice were still present, and the mice appeared to remain free from the devastating effects of the toxic amyloid-beta buildup in the brain.
“The results are surprisingly durable,” says Battaglia. “The rapid drop in amyloid levels shows that once the barrier’s transport system is restored, the brain can efficiently clear harmful proteins on its own. Even months later, we see better memory performance and brain function in treated animals. This suggests that reactivating the brain’s natural repair mechanisms can have long-term benefits, rather than just temporary effects.”
Treating Alzheimer’s Disease in Humans
There is currently no way to cure Alzheimer’s disease. A few drugs can slow its progression and help with the management of symptoms. Accordingly, no therapeutics have come close to the dramatic effects on Alzheimer’s disease as shown in this study.
All the same, there could be a long road ahead before translating these findings to humans. Battaglia and colleagues’ study provides early-stage, preclinical evidence for the effectiveness of polymersome nanoparticles, but translating these findings to make an Alzheimer’s therapy for humans could take 10 to 12 years, if all goes well with its development. However, Battaglia has an optimistic outlook.
“There’s still important work ahead before human testing, but the results give us strong hope,” says Battaglia. “Our next steps involve confirming safety and reproducibility in larger preclinical models and conducting detailed toxicology studies in accordance with regulatory standards. If these confirm what we’ve already seen, a strong recovery with no toxicity, we plan to move toward early clinical trials. The goal is to bring this approach from the lab to patients, offering a new way to treat Alzheimer’s by restoring the brain’s own defense and repair systems.”
For the time being, this study may give a glimmer of hope—the data from Battaglia and colleagues’ study could offer a glimpse of what a true Alzheimer’s disease cure looks like.
Model: APP/PS1 mice (12 months old)
Dosage: 200 μL of A40-POs polymersomes (10 g/L) via intravenous injection

