Scientists Find a New Way to Stop Dangerous Artery Plaques From Growing
A new study shows cyclodextrin reduces artery plaques in mice by lowering cholesterol and blocking inflammatory cell death.
Highlights
- A sugar-derived compound called cyclodextrin shrank artery plaques in mice.
- Cycloextrin lowered cholesterol, reduced inflammation, and kept artery cells healthier.
Atherosclerosis, the gradual buildup of fatty plaques in arteries, is a leading cause of heart attacks and strokes. While current treatments focus on lowering cholesterol or controlling blood pressure, many patients still develop severe disease. Scientists are now focusing their attention on the inflammatory processes that drive plaque growth and rupture.
One major suspect is pyroptosis, a form of programmed cell death that ignites inflammation. Unlike programmed cell death (apoptosis), which quietly dismantles a cell, pyroptosis ruptures the cell membrane, releasing inflammatory molecules such as IL-1β and IL-18. At the center of this process is gasdermin D (GSDMD), a protein that, once activated, punches pores in membranes and triggers cell swelling and rupture.
A new study in Scientific Reports suggests that methyl-β-cyclodextrin (Mß-CD), a compound derived from sugar molecules, can suppress this destructive cascade. By blocking GSDMD-mediated pyroptosis, Mß-CD eased plaque formation and inflammation in mice prone to atherosclerosis.
Cyclodextrin Reduces Plaques and Inflammation
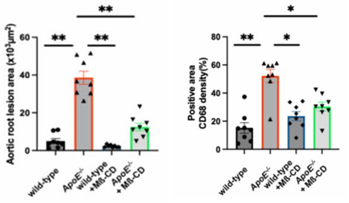
Researchers fed ApoE−/− mice (a standard model for atherosclerosis) a high-fat diet for 12 weeks. As expected, the animals developed large plaques in their aorta. But when treated twice weekly with Mß-CD, the plaque area shrank by more than half, from nearly 20% of the aorta to just 8%.
The therapy also lowered the number of infiltrating macrophages, immune cells that drive inflammation inside plaques. Blood tests revealed reductions in harmful lipids like LDL cholesterol and triglycerides, alongside increases in protective HDL cholesterol. Pro-inflammatory cytokines IL-1β and IL-18 also dropped with treatment, pointing to a broad anti-inflammatory effect.
Stopping Inflammatory Cell Death at Its Root
The researchers found that Mß-CD interrupts the chain reaction that normally pushes cells toward pyroptosis, a fiery form of cell death that fuels plaque growth.
Here’s how it usually works: oxidized LDL, a chemically altered form of LDL cholesterol that becomes especially harmful once it’s damaged and builds up in artery walls, acts like a spark that sets off inflammatory alarm signals inside cells. Those signals eventually switch on gasdermin D, a protein that drills holes in the cell’s membrane. Once that happens, the cell bursts open, spilling inflammatory molecules and worsening artery damage.
In both mice and cultured artery cells, this destructive cascade fired up strongly when oxidized LDL was present. But when Mß-CD was added, the signals quieted down, and gasdermin D never got the chance to punch holes, leaving the cells intact instead of inflamed.
Microscope images confirmed the effect: cells exposed only to oxidized LDL glowed red with a dye that marks ruptured membranes, while cells pretreated with Mß-CD stayed mostly whole. This showed that the compound preserved cell health and blocked the inflammatory death that drives atherosclerosis.
Cyclodextrin’s Potential Against Heart Disease
The study shows that methyl-β-cyclodextrin may help fight atherosclerosis in two important ways. It reduces the buildup of cholesterol and it prevents pyroptosis, the inflammatory cell death that weakens artery plaques. Together, these effects make plaques smaller and more stable, lowering the chance of a rupture that can trigger a heart attack or stroke.
The findings are early and come from mice and rat cells, so more work is needed to know if the same results apply to people and what doses would be safe. Pyroptosis is also only one of several types of cell death linked to atherosclerosis, so future research will need to explore how it interacts with other processes such as apoptosis and necrosis.
Even with these caveats, the results are encouraging. Cyclodextrins are already used in food and medicine to improve how drugs dissolve, which could make it easier to test them in clinical settings. By addressing both cholesterol buildup and inflammation, Mß-CD points toward a new therapeutic strategy that may offer stronger protection against cardiovascular disease.
Model: Male ApoE−/− mice fed a high-fat diet for 12 weeks to induce atherosclerosis.
Dosage: Methyl-β-cyclodextrin (Mß-CD) at 2.0 g/kg, subcutaneous injection, twice a week

