Scientists Find Rapamycin Inhibits Bone Loss by Increasing Mitochondrial Quality
Scientists show that age-related bone loss can be recovered by the immunosuppressant drug rapamycin, which promotes mitochondrial health and reduces bone cell death in mice.
Highlights:
- Rapamycin heightens mitophagy — mitochondrial quality and quantity control — in a mouse model for bone aging.
- Bone cell death is reduced by rapamycin treatment.
- Treatment with rapamycin increases bone mineral density, volume, and thickness.
As our mitochondria work hard to generate the cellular energy we need to stay alive, they produce reactive oxygen species (ROS) — short-lived molecules that are normally quenched by our cell’s antioxidant defenses. When mitochondria become damaged, as with aging, they produce excessive ROS, causing oxidative stress — damage to the DNA, proteins, and fats of our cells. This oxidative stress leads to the generation of toxins called advanced oxidation protein products (AOPPs). AOPPs are the latest marker for oxidative stress that researchers from Southern University in China have utilized to induce bone aging in mice.
As reported in Cell Death & Disease, Li and colleagues use the longevity-associated medication rapamycin to reduce the effects of AOPPs on mouse bones. The researchers show that rapamycin increases mitophagy in the tibia bone of mice, which reduces AOPP-induced bone cell death. Rapamycin also prevents AOPP-induced reductions in bone mineral density, thickness, and volume.
“Our study indicated that therapeutic strategies aimed at upregulating [bone cell] mitophagy and preserving mitochondrial function might have potential for treating age-related osteoporosis,” state Li and colleagues.
Rapamycin Prevents Osteoporosis
One of the underlying cellular processes that drive aging is a decrease in autophagy — the cellular waste and disposal system. Autophagy encompasses mitophagy — the removal of unhealthy mitochondria. Li and colleagues found that exposing mice to AOPPs increases mitophagy, as measured by mitophagy-related proteins in the tibia bone. Moreover, injecting the mice for 16 weeks with 1 mg/kg/day of rapamycin increases mitophagy further.
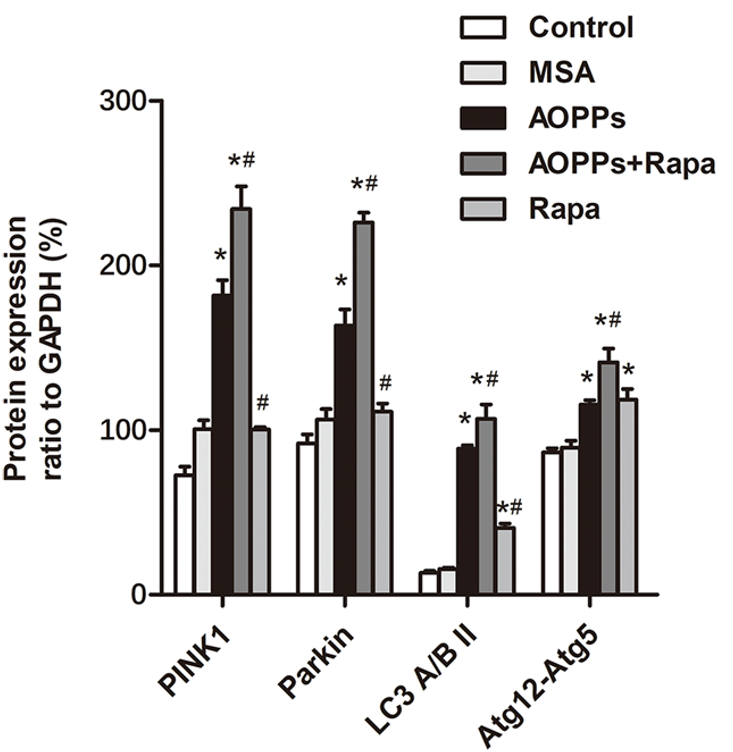
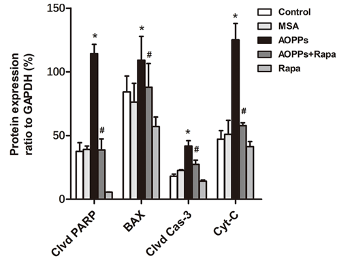
Unhealthy, damaged mitochondria are the primary source of cellular ROS and oxidative stress. If ROS production cannot be quenched by mitophagy and other cell survival processes, oxidative stress eventually leads to cell death. Li and colleagues found that AOPP exposure increases cell death-related proteins in the mouse tibia. However, this increase was subdued by rapamycin treatment.
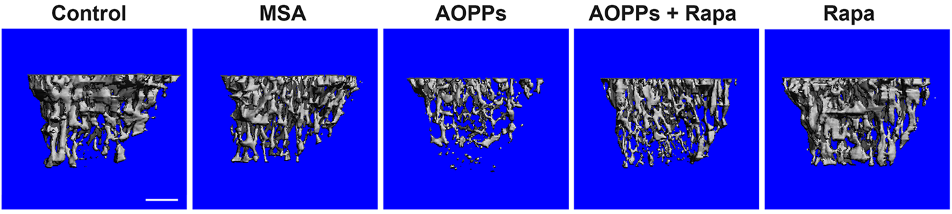
Because cells are the foundational unit of our organs, cell death (without regeneration) causes progressive cell-by-cell organ deterioration, manifesting in what we call disease. In the case of bone deterioration, this disease is called osteoporosis — bone fragility caused by loss of bone mass and density. Osteoporosis is an age-related disease that increases the risk of bone fractures, including hip fractures in older individuals.
Li and colleagues found that AOPPs decrease bone volume, thickness, and density in mice, demonstrating that AOPPs can cause osteoporosis in mice. By treating these mice with rapamycin, it was shown that bone volume, thickness, and density were largely restored. These findings suggest that rapamycin can help prevent or treat osteoporosis.
Similar to the mouse experiments, experiments with mouse bone cells in a dish (in vitro) showed that AOPPs increased ROS, mitophagy, and cell death. Furthermore, rapamycin further increased mitophagy while reducing ROS and cell death. Genetically removing the proteins responsible for mitophagy eliminated the affects rapamycin, suggesting that rapamycin likely prevents osteoporosis by increasing mitophagy to levels that save bone cells from death.
Rapamycin: The Aging-Intervention Medicine?
Rapamycin has previously been shown to mitigate cognitive impairment and muscle weakness in aged mice. Indeed, rapamycin increases the lifespan of mice by 10%, but at the expense of growth when administered early in life. Therefore, it seems that rapamycin may serve to counter aging and increase lifespan in fully developed adults. Still, it remains to be seen how rapamycin affects longevity in humans.
Model: Mice
Dosage: 1 mg/kg/day of rapamycin by intraperitoneal injection for 16 weeks

