Selenium Mimics Exercise, Doubles New Neurons, and Reverses Memory Loss
The cheap, readily available dietary supplement mimics the positive effects of exercise on the brain and could impact people with limited mobility.
Highlights
- By mimicking the effect of exercise, selenium increases new neurons (neurogenesis) two-fold in the aging brain of mice.
- In mice, selenium reverses the cognitive decline associated with aging and brain injury.
- Selenium may boost cognition in individuals who cannot exercise due to advanced age, frailty, or disability.
Exercise has often been touted as the best medication for the brain, as habitually breaking a sweat has been shown to boost mood and neurogenesis – when the brain makes new neurons. But, with age or injury, many people simply cannot work out and reap the benefits of exercise.
Research published in Cell Metabolism by a research team from Germany and Australia shows that the trace element selenium mimics exercise to restore neurogenesis and reverse the cognitive decline associated with aging and brain injury. Since selenium is a cheap, readily available dietary mineral found in several commonly eaten foods like nuts, grains, and dairy products, the mineral could easily be supplemented in the diets of people with reduced mobility, whether due to aging, injury, or disease.
“It’s the first time a substance that is usually in the diet has been found to have such a relevant and clear effect on neurogenesis,” says Juan Encinas, a neurobiologist at the Achucarro Basque Center for Neuroscience.
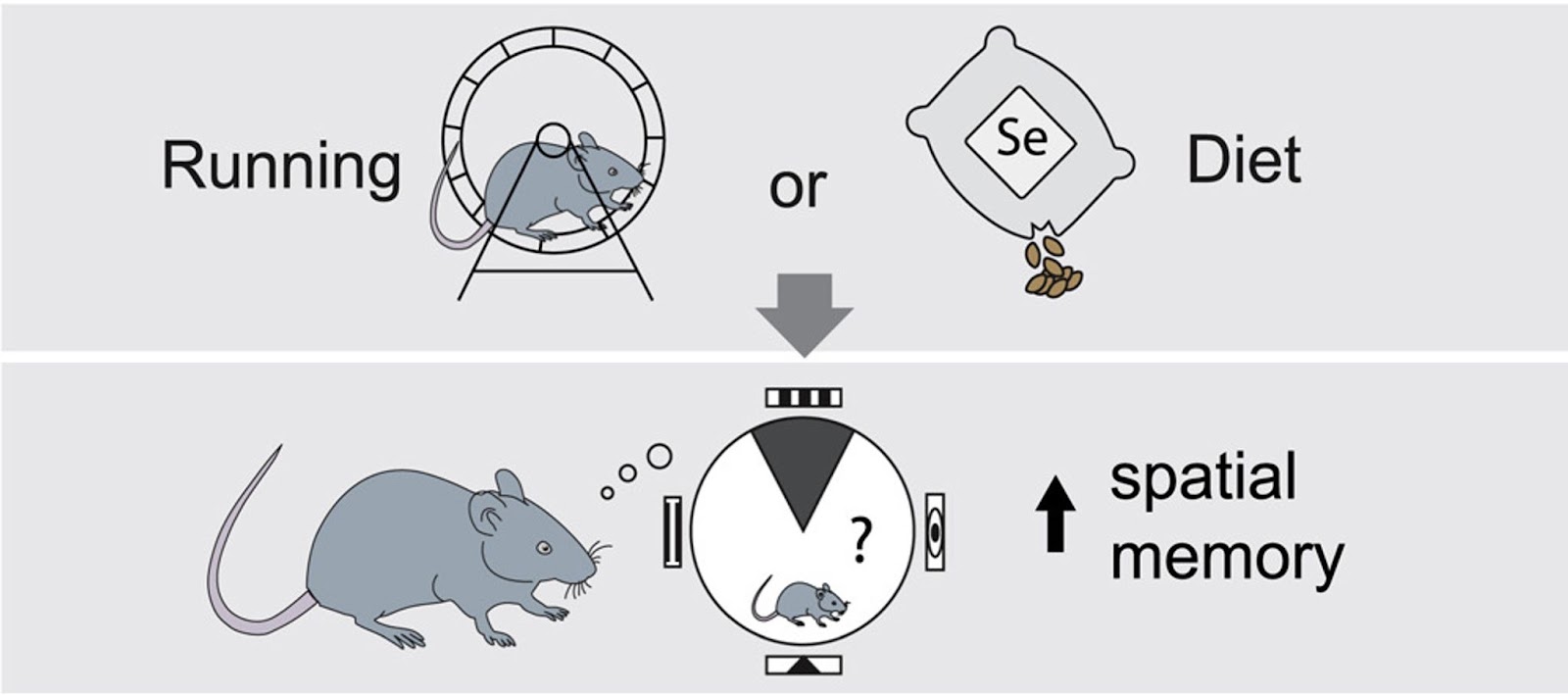
How Does Exercise Boost Neurogenesis?
Adult neurogenesis underlies our ability to continually learn and create memories throughout our lives. There are two major niches of adult neural stem cells in the brain: the hippocampal dentate gyrus and the subventricular zone of the lateral ventricles. These two regions share many common features, but it is becoming increasingly clear that they are under distinct regulatory control.
One key difference between these two neurogenic niches’is their susceptibility to the induction of proliferation in response to physiological stimuli, such as physical exercise. The broad range of effects of exercise across the body makes it likely that systemically released factors in the blood could serve as systemic mediators of the neurogenesis-promoting effect. Although the neurogenesis-enhancing effects of exercise have been known for more than two decades, the molecular mechanisms underlying this response have remained largely unclear.
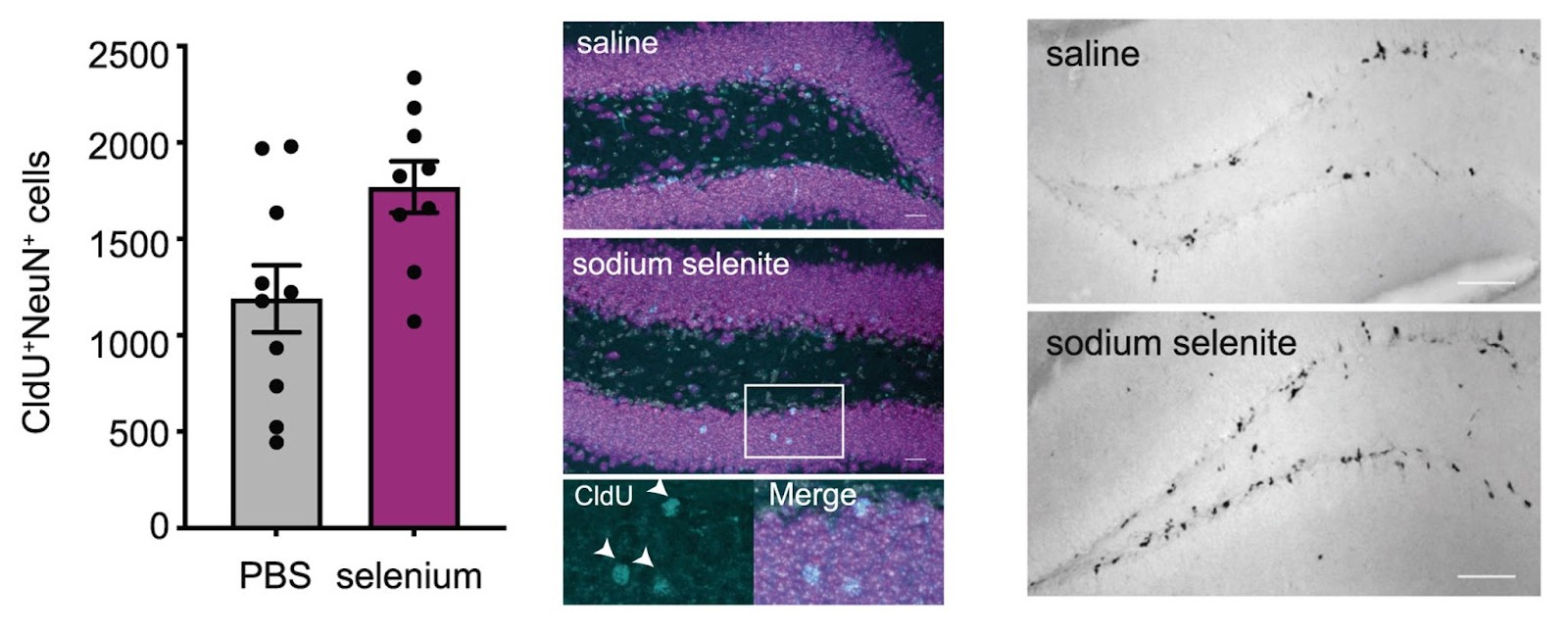
Selenium Increases Adult Neurogenesis in Mice
In this study, Leiter and colleagues sought to identify systemic mechanisms by which exercise regulates adult hippocampal neurogenesis. Upon performing a screen for proteins in the blood plasma from daily exercised mice, the researchers identified 68 proteins with significant running-induced changes in their plasma levels. Of these, selenoprotein P1 (SEPP1) was one of the most significantly upregulated proteins, present in the plasma of running mice at more than twice the level of the control mice that were not exercised. While studies have identified 25 mammalian selenoproteins so far, SEPP1 is the most important for maintaining selenium levels in the brain. The activity-dependent increase in blood SEPP1 levels suggested a potential role of selenium in activating quiescent neural progenitor cells (NPCs).
Leiter and colleagues then show that exercise increases the transport of selenium from the blood to NPCs of the hippocampal dentate gyrus by elevating the systemic level of SEPP1. These data show that an exercise-induced increase in selenium transport activates hippocampal NPCs, resulting in increased NPC replication and adult neurogenesis. Along these lines, Leiter and colleagues found that similar to exercise, selenium supplementation increased NPC proliferation in the dentate gyrus of the hippocampus but not in the subventricular zone or in areasesustained by hippocampal injury in aged mice. The international research team proposes that this finding explains why blood components that are known to be changed by exercise exclusively affect hippocampal neurogenesis without influencing proliferation in the subventricular zone.
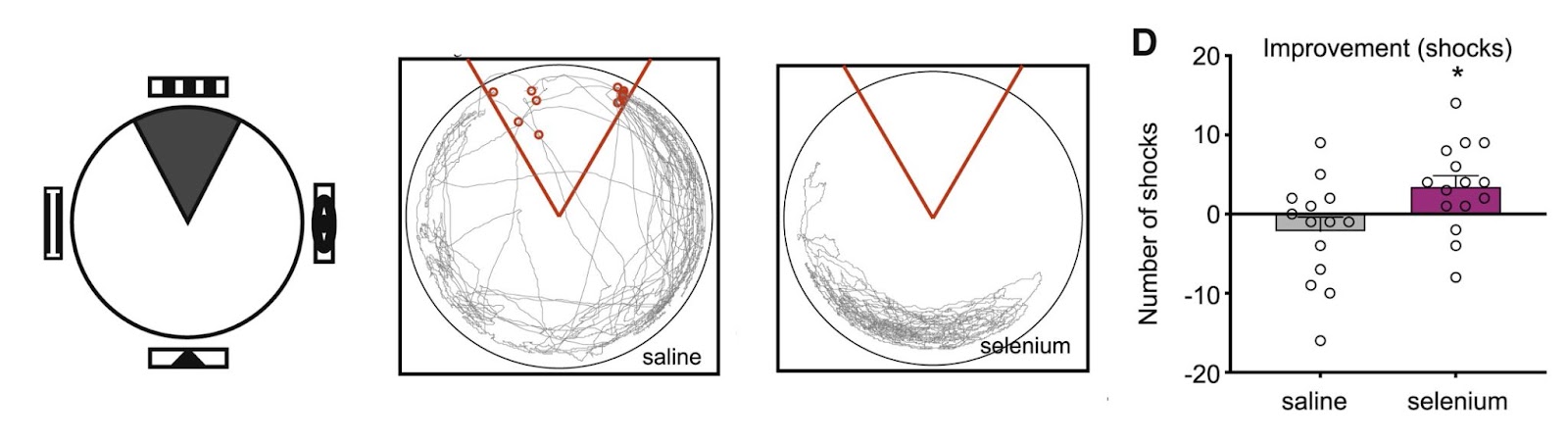
Selenium Reverses Learning Deficits Induced by Hippocampal Injury and Aging
The generation of new brain cells had significant effects on the cognitive abilities of mice. Using two different tasks to evaluate spatial learning and memory, the international research team found that supplementing water with selenium (50 nM) can restore age-related or injury-induced deficits in hippocampal function. In the first task, the mice learned to avoid a stationary shock zone using visual cues as a reference. In the second, Leiter and colleagues placed mice in the center of a brightly lit table with 32 evenly spaced holes around the edges. The mice then used the visual cues placed around the room to find the one hole with an escape chamber beneath it.
The finding that selenium metabolism is involved in mediating exercise-induced increases in adult hippocampal neurogenesis demonstrates how systemic or environmental factors can regulate adult neurogenesis and hence plasticity in the hippocampus.
“I’ve been working on neurogenesis for almost 20 years … and we’ve never seen anything like that before,” says Tara Walker, a neuroscientist at the University of Queensland’s Brain Institute. Walker says, “This is the first study to show that selenium supplementation mediates exercise-induced adult neurogenesis and reverses injury- and aging-induced learning deficits.”
This could have far-reaching implications, as the activity-dependency of adult hippocampal neurogenesis is one of its key features and central to modern concepts of how adult-generated neurons provide life-long adaptability to the hippocampus in both health and disease. The identification of the mechanism underlying the exercise-induced increase in adult neurogenesis could facilitate the discovery of novel therapeutic interventions (including dietary selenium supplementation), which could be used to mimic the beneficial effects of exercise on cognitive function.

