Senolytic Drug Halts GI Cancer Progression: New Study Shows
A new study shows the senolytic drug ABT-263 reduces radiation-induced gastrointestinal tumors in mice by clearing senescent cells and suppressing cancer-promoting inflammation and signaling pathways.
Highlights
- ABT-263 (Navitoclax), a senolytic drug, reduced both precancerous and cancerous intestinal tumors in mice exposed to radiation.
- The treatment worked by removing senescent cells—damaged cells that drive inflammation—and lowering signals that promote tumor growth.
A new preclinical study published in Aging provides further evidence that removing certain damaged cells, called senescent cells, can reduce the risk of radiation-induced gastrointestinal (GI) cancers. Senescent cells are aged, dysfunctional cells that have stopped dividing but stubbornly remain in tissues, where they secrete harmful molecules that can promote inflammation and cancer. Researchers from Georgetown University demonstrated that ABT-263 (also known as Navitoclax), a type of drug called a senolytic agent, significantly reduced both benign and malignant intestinal tumors in a mouse model predisposed to develop intestinal cancers after radiation exposure.
These findings offer proof of concept that clearing radiation-induced senescent cells can help reduce the cancer-friendly environment created by chronic inflammation and cancer-promoting signals.
Radiation Leaves Behind More Than DNA Damage
Radiation exposure, whether from medical treatments like radiation therapy, environmental sources, or space travel, is known to increase the risk of various cancers, including those affecting the GI tract. While much of this risk has traditionally been blamed on direct DNA damage, this study highlights the role of senescent cells as lingering drivers of cancer following radiation exposure.
Even though these cells no longer divide, they secrete a mix of inflammatory molecules known collectively as the senescence-associated secretory phenotype (SASP). This SASP cocktail promotes tissue breakdown, immune dysfunction, and ultimately, tumor formation. The study’s authors hypothesized that clearing these senescent cells after radiation exposure might reduce the risk of GI cancer.
ABT-263 Removes Senescent Cells and Reduces Tumor Development
To test this, the researchers used Apc1638N/+ mice, which are genetically predisposed to developing intestinal tumors, making them a model for studying human-like colorectal cancers.
The mice were exposed to either 2 Gy gamma rays (representing low-LET radiation, similar to medical X-rays) or 0.1 of Gy 28Si ions (representing high-LET radiation, similar to the cosmic rays encountered in space). After a two-month delay (allowing the senescent cells to accumulate), the mice received oral ABT-263 for five days a week over a three-month period.
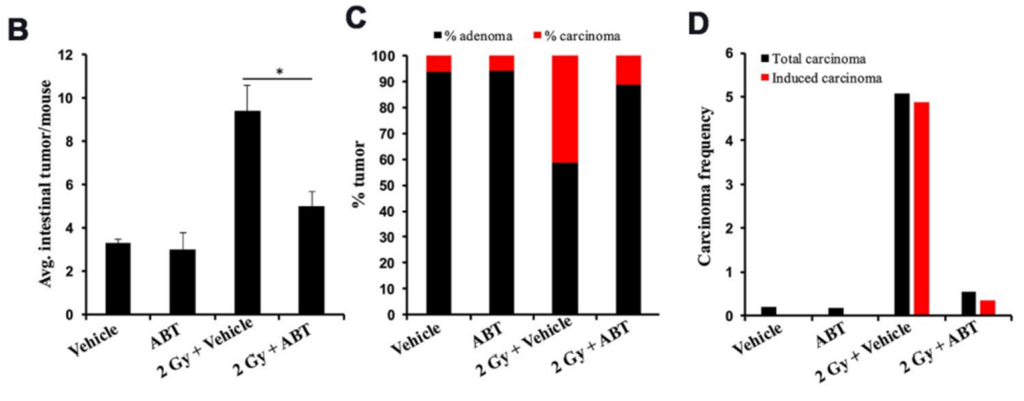
At five months post-exposure, tumor assessments showed a significant reduction in both precancerous adenomas and malignant carcinomas in ABT-263-treated mice compared to untreated controls. Importantly, ABT-263 had no effect on spontaneous tumors in unirradiated mice, suggesting its benefits are specific to the radiation-damaged tissue environment.
Tissue analysis confirmed that ABT-263 effectively cleared intestinal cells marked by both p16 (a hallmark of senescence) and IL-6 (a SASP factor), demonstrating its senolytic activity directly in the gut tissues.
ABT-263 Also Reduces Inflammation and Cancer-Promoting Signals
The researchers also examined the drug’s effects beyond the intestines. Blood tests showed that six major pro-inflammatory and cancer-promoting molecules (including TNFRSF1B, CCL20, and CXCL4) were elevated after radiation exposure but significantly reduced following ABT-263 treatment.
At the molecular level, ABT-263 suppressed activation of the β-catenin pathway—a well-known driver of colorectal cancer—along with its key downstream protein, cyclin D1. These findings suggest that ABT-263 not only removes harmful senescent cells but also disrupts important pathways that contribute to tumor growth after radiation exposure.
Limitations and Future Directions
While these findings are promising, the authors acknowledge that ABT-263 is associated with known side effects, particularly thrombocytopenia (low platelet count), which limits its clinical utility for long-term preventive use in otherwise healthy individuals. Nonetheless, this study provides strong preclinical evidence that senolytic strategies targeting senescent cells represent a viable approach to reduce radiation-induced GI cancer risk.
Future studies will be essential to explore safer, more selective senolytic agents, such as dasatinib and quercetin, fisetin, or SASP-neutralizing monoclonal antibodies, which may offer similar cancer-preventive benefits with improved safety profiles. Additionally, further research is needed to define the optimal timing, dosage, and duration of post-exposure senolytic interventions, particularly in high-risk human populations such as cancer survivors, astronauts, and individuals exposed to environmental radiation.
By focusing on the removal of senescent cells and suppression of their cancer-promoting secretions, this study demonstrates the potential for senolytic interventions to complement existing cancer prevention strategies, especially in scenarios where radiation exposure cannot be avoided or mitigated by shielding alone. Collectively, these findings open new avenues for addressing the long-term health consequences of radiation exposure by targeting the tissue and immune environment that fuels cancer progression.
Model: Apc1638N/+ mice
Dosage: 50 mg/kg oral ABT-263 for five days a week over a three-month period

