A Small Molecule That Activates NAMPT Boosts NAD+ Levels
SBI-797812 turns NAMPT into a “super catalyst” that more efficiently generates NMN
Pharmacological strategies that boost intracellular NAD+ are highly coveted for their therapeutic potential. One approach is the activation of nicotinamide phosphoribosyltransferase (NAMPT) to increase the production of nicotinamide mononucleotide (NMN), a predominant NAD+ precursor in mammalian cells. Using tiny, synthetic pharmaceuticals called small molecules that can activate NAMPT is a pioneering approach to raise intracellular NAD+ and realize its associated salutary effects.
Gardell and colleagues from the Sanford Burnham Prebys Medical Discovery Institute published an article in Nature Communications that set out to identify small molecule NAMPT activators. By screening thousands of molecules, they identified SBI-797812, which activated purified NAMPT, raised NMN and NAD+ levels in cultured cells, and boosted NAD+ in the livers of mice. “A small-molecule NAMPT activator exemplified by SBI-797812 represents a pioneering pharmacological approach to raise intracellular NAD+ and realize diverse and potentially impactful therapeutic benefits,” said the authors in their article.
How to harness the NAD+ synthesis pathway
NAD+ plays a vital role in diverse cellular processes that govern human health and disease. It has been implicated in cellular processes including cell signaling, DNA repair, cell division, and epigenetics—changes in heritable characteristics that do not involve alterations in the DNA sequence. Elevated tissue levels of NAD+ have been linked to salutary effects including healthy aging. Thus, there is a keen interest in pharmacological and nutraceutical strategies to boost intracellular NAD+ levels.
In mammalian cells, the principal contributor to NAD+ synthesis is the nicotinamide (NAM) salvage pathway. This involves the sequential actions of nicotinamide phosphoribosyltransferase (NAMPT) and NMN adenylyltransferase (NMNAT1-3). NAMPT forms NMN from NAM, and NMNAT1-3 produces NAD+ from NMN. For these reasons, many pharmacological approaches to boost intracellular NAD+ levels focus on discovering compounds that increase the activity of NAMPT.
Discovery of SBI-797812, a small molecule NAMPT activator
A chemical library (57,004 compounds) was screened for small molecules that bound to human NAMPT. Five hundred fifteen compounds (0.9%) were identified as NAMPT binding molecules. While the majority of the hits were inhibitors or had no activity, 30 compounds (5.8%) were NAMPT activators. This search for small molecules that stimulated NAMPT-mediated NMN formation yielded SBI-797812.
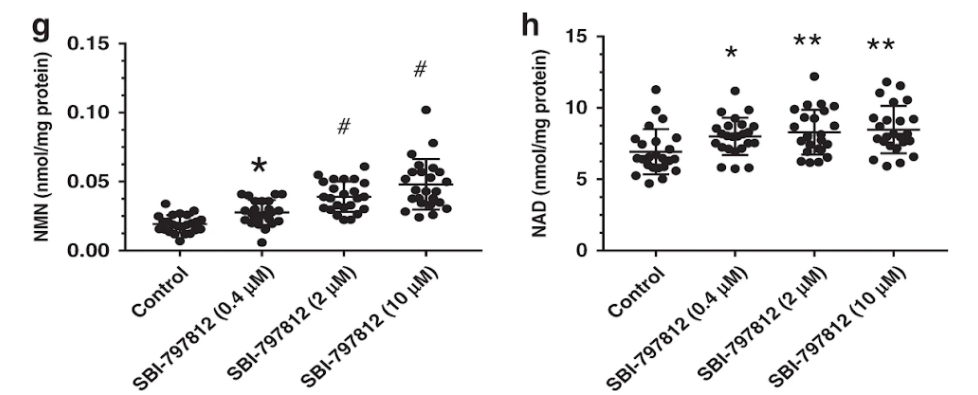
SBI-797812 is a compound that is structurally similar to active-site directed NAMPT inhibitors and blocks the binding of these inhibitors to NAMPT. These effects of SBI-797812 turn NAMPT into a “super catalyst” that more efficiently generates NMN. To show this, Gardell and colleagues first established that SBI-797812 can activate purified NAMPT floating around in a test tube.
Next, they tried to see if SBI-797812 could also activate NAMPT in cultured cells. SBI-797812-treated A549 cells had a major increase in NMN (17.4-fold) that proportionally was much larger than the increase of NAD+ (2.2-fold). But the total NAD+ reached much higher levels than the total NMN rise by approximately 17.5-fold.
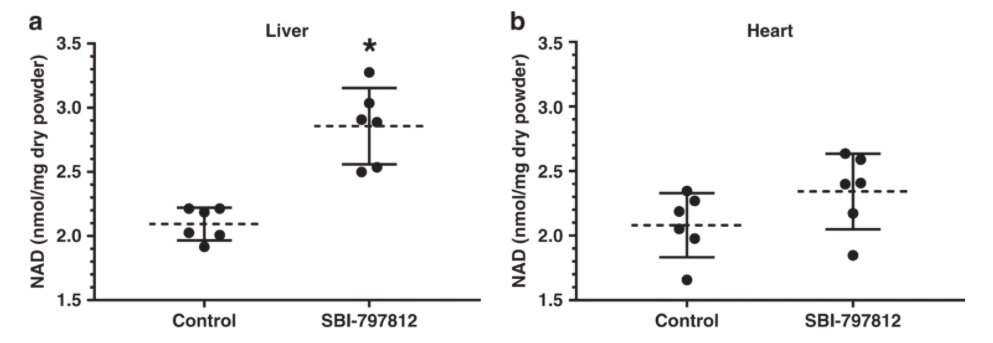
Although SBI-797812 was designed to target human NAMPT, the researchers wanted to see how this NAMPT activator worked in live animals. To do so, mice were dosed with SBI-797812 (20 mg kg−1 i.p.) and liver, heart, gastrocnemius muscle, and quadriceps muscle were harvested after 4 h.
Despite the transient plasma exposure of SBI-797812 and lower apparent affinity of SBI-797812 for mouse NAMPT, a significant 1.3-fold increase of NAD+ was detected in the liver. There was also a trend towards increased NAD+ levels in cardiac tissue. Skeletal muscle, either gastrocnemius or quadriceps, did not exhibit increased NAD+ levels after dosing with SBI-797812. Hence, the liver which displayed a statistically significant increase of NAD+ after SBI-797812 dosing exhibited the highest level of the compound.
Small molecules versus NAD+ precursors
Future studies with superior compounds will allow researchers to decipher if the relatively modest in vivo NAD+ elevating effects of SBI-797812 can be improved with analogs that exhibit more favorable pharmacokinetics and/or potency versus murine NAMPT. Besides, changes in the study design such as longer duration of dosing and interrogation of other tissues may also unveil more robust effects of a NAMPT activator.
Comparing the therapeutic utility of small molecule NAMPT activators to NAD+ boosters like NR or NMN will be essential. The fact that SBI-797812 acts to promote NAD+ synthesis along with its ability to suppress feedback inhibition of NAMPT activity by NAD+ are two discriminating attributes that are likely to be advantageous. In any event, a small molecule NAMPT activator exemplified by SBI-797812 represents a pioneering pharmacological approach to raise intracellular NAD+ and realize diverse and potentially impactful therapeutic benefits.

